| Cite this article as: | Xiao-li Xi, Ming Feng, Li-wen Zhang, and Zuo-ren Nie, Applications of molten salt and progress of molten salt electrolysis in secondary metal resource recovery, Int. J. Miner. Metall. Mater., 27(2020), No. 12, pp.1599-1617. https://doi.org/10.1007/s12613-020-2175-0 |
Molten salt is an excellent medium for chemical reaction, energy transfer, and storage. Molten salt innovative technologies should be developed to recover metals from secondary resources and reserve metals from primary natural sources. Among these technologies, molten salt electrolysis is an economic and environment-friendly method to extract metals from waste materials. From the perspective of molten salt characteristics, the application of molten salts in chemistry, electrochemistry, energy, and thermal storage should be comprehensively elaborated. This review discusses further directions for the research and development of molten salt electrolysis and their use for metal recovery from various metal wastes, such as magnet scrap, nuclear waste, and cemented carbide scrap. Attention is placed on the development of various electrolysis methods for different metal containing wastes, overcoming some problems in electrolytes, electrodes, and electrolytic cells. Special focus is given to future development directions for current associated processing obstacles.
The electrode potential of some active metals, including aluminum, sodium, magnesium, lithium, and other alkali and alkaline earth metals, is more negative than that of hydrogen. Hydrogen gas is generated preferentially on the cathode during the electrolysis of aqueous solutions because of the small overvoltage of hydrogen evolution. Therefore, light metals cannot be extracted or refined by aqueous solution electrolysis. Extracting active metals from molten salts has become the only dominant production method. The significant achievements of molten salts in extracting metals are well recognized worldwide [1–5]. Molten salts have been used for the industrial production of aluminum, magnesium, and other light metals from as early as the 19th century. Later, they have also been used for the production of rare elements, thorium, uranium, tantalum, and other metals [6–8]. The growing interest in the energy applications of molten salts is justified by their comprehensive properties. Molten salts have been widely used in electrolytic metallurgy, synthetic compound materials, cells, energy storage, and so on because of their excellent electrical conductivity, high electrochemical stability, high heat conductivity, low viscosity, high density, relative stability at high temperatures, and high heat capacity per unit volume [9–11].
With the developments in society, productivity, and technology, the demands for metals continue to increase. The global needs for metal resources are expected to double between 2010 and 2030 than before [12–14]. Excessive metal mining has led to increasingly serious problems, resulting in a tight supply of many metal resources. The extensive use of mineral resources is threatening the natural sources in the near future. Recycling metals from secondary resources not only makes up for the lack of mineral resources of the original metals but also reduces the cost of materials and metal ore mining. Recycling metals from secondary resources also contributes to a virtuous cycle of sustainable, low-carbon, and efficient utilization of natural resources [15–17].
Traditional methods for the treatment of secondary resources consist of burying, incinerating, high-temperature pyrolysis, and smelting processes. Despite the research efforts exerted to develop effective secondary resource recovery methods over the years, traditional methods still have many problems, such as low cost-effectiveness and environmental pollution [18–21]. Therefore, a new method and technology should be urgently developed to recover metals from secondary resources effectively. Molten salt electrolysis, which converts electric energy into chemical energy by using molten salts as the electrolyte for metal extraction, has received widespread attention. The impressive achievements of molten salts in extracting metals are well recognized worldwide. With the rapid development of molten salt electrochemistry, molten salt electrolysis has played a far greater role than expected in areas such as energy supply, recycle utilization of resources, and metal smelting. It is not only an innovative, economical, and green approach for recycling metals but also an important research direction for the development of recycling technology [22–23].
As a high-temperature melt, molten salts feature high heat capacity, high electrochemical stability, excellent heat stability, high electric conductivity, and low cost. Molten salts are an excellent medium for chemical reaction, energy transfer, and storage and has become a highly recognized electrolysis medium.
Molten salt can be used as a chemical reaction medium for catalysis, cracking, halogenation, preparation of semiconductors, inorganic powders, and surface functional coating materials. In the molten salt reaction medium, the reaction proceeds at the atomic level during the preparation of materials by using molten salts, enhancing the fluidity and remarkable increasing the diffusion rate. Common molten salts include sulfates, carbonates, and halides. Compared with traditional methods, such as solid-phase method, sol–gel method, co-precipitation method, and hydrothermal method, the use of molten salts to prepare materials effectively decreases the synthesis temperature, shortens the reaction time, and controls the composition, morphology, and powder characteristics as evidenced by their low cost, easy operation, short heat preservation time, and so on [24–26].
Since Arendt [27] first produced BaFe12O19 and SrFe12O19 from molten salts in 1973, the molten salt method has been used to prepare materials for various applications. Afterward, various inorganic powder materials, including SrBi4Ta4O15, SrBi2Ta2O9, Bi4Ti3O12, and Na0.5Bi4.5Ti4O15, have been prepared using the molten salt method. The synthesis of powders by the molten salt method can be divided into two processes: powder particle formation and growth process. Thus, the morphology of powders is initially controlled by the formation process and then by the growth process. In general, the synthesis mechanism can be divided into two categories. (1) When the mobility of the oxides in the molten salt is higher than that in the solid phase, diffusion and mixing for the reaction occur in a short time. Precipitation occurs after exceeding its solubility in molten salt. (2) When the solubility of the target oxides is greater than those of other nontarget oxides in the molten salt, the target oxides diffuse to the surface of the nontarget oxides and finally generate the corresponding products on the surface [28–29].
In addition to inorganic powder materials, molten salts are also used to prepare intermetallic compounds, surface treatments, zirconium fluoride glass, high-temperature superconductor single crystal, and lithium niobate single crystal materials.
Molten salt plays an important role as an electrolyte medium in electrochemistry. At present, most metals are extracted from the minerals of crustal deposits. Primary metals are mainly extracted using mining, flotation, hydrometallurgy, and pyrometallurgy. However, traditional processes cause pollution. Thus, the operation and production capacity should incorporate environmental protection measures. New process methods in advanced industrial setting should be developed to meet stringent environmental protection requirements [30–31].
Molten salts are characterized by a relatively wide electrochemical window, high electrical conductivity, and fast reaction kinetics at high temperatures. In addition, molten salts are more electrochemically stable than aqueous electrolytes. The reduction of metal ions with the hydrogen evolution in solutions decreases the electrochemical current efficiency. The formation of oxide films and hydrides and the stable deposition of oxygenated cations also impede the electrodeposition of metals in aqueous solutions. Molten salt electrolysis provides a conducive working environment for metal processing in electrochemical reactions. It runs smoothly under anhydrous and oxygen-free conditions, effectively inhibiting hydrogen evolution [32]. In the metallurgical industry, molten salt electrolysis has been widely applied to manufacture Al, Mg, rare-earth metals, refractory metals, and other metals. Among them, molten salt-based electrolytic aluminum smelting has achieved large-scale global industrialization and is a green energy-saving system for environmental protection with relatively high production efficiency.
In the early 19th century, British chemist Humphrey Davy first invented molten salt electrolysis, which could produce many types of chemically active metals. Hall-Héroult smelted aluminum in the high-temperature range of 1223–1233 K by using cryolite–Al2O3 molten salts as an electrolyte. This method had a history of more than 100 years from lab-scale to large-scale industrial production. Exploratory investigation has been carried out for the preparation of refractory metals by using aluminum electrolysis with traditional molten salts. Molten salt electrolyte systems, such as K2TaF7–Ta2O5/KCl–KF, K2NbOF5/KCl, K2TaF7/KCl–NaCl, and K2NbF7/KCl–NaCl, have been developed to extract niobium and tantalum. K2HfF6/KCl–NaCl, K2ZrF6/KCl–NaCl, and TiCl4/LiCl–KCl electrolyte systems for hafnium, zirconium, and titanium, and other electrolyte systems have also emerged [33–35].
Titanium transition metal has been investigated in detail; however, many problems still exist. Titanium smelting was initially based on the Kroll and Hunter methods operated intermittently; however, both methods entail high cost and cause serious pollution. Molten salt electrolysis is superior to the Kroll and Hunter methods from the perspective of reducing energy consumption and simplifying the process [36–37]. Ginatta et al. [38–40] extensively studied the traditional TiCl4 electrolysis process; however, titanium production using this method is all-time high. In addition, the traditional molten salt electrolysis has major problems, such as low solubility of TiCl4 in the molten salt, high electrolysis temperature, large energy consumption, low current efficiency, many side reactions, and low energy utilization.
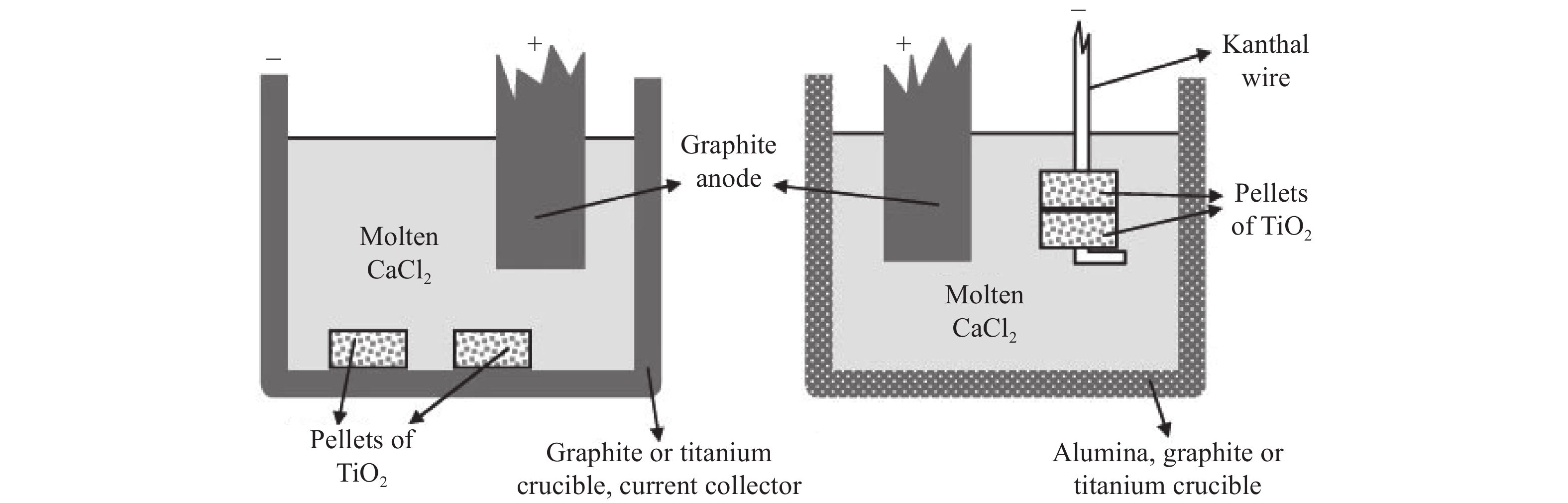
The FFC (Fray-Farthing-Chen) Cambridge process developed by Cambridge University solved the above problems for the electro-deoxidation of solid metal oxides, as shown in Fig. 1 [41]. The FFC Cambridge process has its own characteristics, such as shorter process cycle, lower cost, and more environmental friendly compared with the traditional Kroll method. The raw material is a non-volatile oxide, which simplifies the preparation process in comparison with TiCl4 molten salt electrolysis. This process has attracted global interest owing to its simplicity and rapidity as well as its applicability to a wide range of metal oxides. The method was also extended to other metals and non-metals, such as Mg, Cd, Nb, Ta, Zr, Hf, V, W, and Si [42–44]. The FFC process was commercialized by British company Metalysis Ltd. and has been proven to produce a large range of metals (e.g., Cd, Ta, and Zr) and alloys (e.g., NbTi, NiTi, and NdFeB) at the industrial scale. In 2018, the industrial-scale production of metal oxides, such as titanium dioxides, tantalum oxides, and uranium oxides, was gradually achieved from the initial kilogram production [45].
Aside from the FFC process, Solid Oxygen-ion Membrane (SOM), Materials and Electrochemistry Research (MER), Quebec Iron and Titanium (QIT), and University of Science and Technology Beijing (USTB) methods have been developed, providing new vitality for the research of molten salt electrolysis, especially for the preparation of metals. The comparison between different Ti electrolysis methods is shown in Table 1.
| Method | Melt | Cathode reaction | Anode reaction | Overall reaction | Temperature / K | Year |
| FFC | CaCl2 | TiO2+e−→Ti +2O2− | 2O2−→O2+4e− | TiO2→Ti+O2 | 1072–1123 | 2000 |
| SOM | CaCl2 –CaF2 | TiOx+2xe−→ Ti+xO2− | O2−+2H2→2H2O+2e− | 2TiOx+2xH2→ 2xH2O+2Ti | 1473–1673 | 2001 |
| MER | NaCl–KCl | Ti3++3e−→Ti | TiO2+C→Ti3++CO2+3e− or TiO2+2C→Ti3++2CO+3e− | TiO2+C→Ti+CO2 or TiO2+2C→Ti+2CO | 1073 | 2002 |
| QIT | CaF2 CaCl2 or CaF2 | TiO2+e−→Ti +2O2− | C+2O2−→CO2+4e− | TiO2+C→Ti+CO2 | 1973–2073 | 2003 |
| USTB | NaCl–KCl | Tin++ne−→Ti (n < 4) | TiCxOy→Tin++CO2+CO+ne− | TiCxOy→Ti+CO2+CO | 1073 | 2006 |
The SOM method is a green and environmental-friendly method first proposed by Boston University for extracting metals and metal alloys from metal oxides. This method overcomes the shortcomings of the traditional method of continuous production, greatly improving the production efficiency and decreasing the production cost. Sponge titanium can be directly extracted from ores containing titanium oxide with short process flow and low energy consumption. The requirements for raw materials are greatly reduced because only TiOx participates in the reaction [46–48]. American Materials and Electrochemistry Research Corporation proposed the MER process using anode dissolution electrolytic reduction to prepare titanium metal. It has the characteristics of easy recovery of excessive reaction heat, automation, and minimal pollution compared with the intermittent Kroll method. The metallic titanium product obtained by this process has less impurity, thus realizing the rapid and continuous production of metallic titanium powders.
The QIT process was developed by the Quebec Iron and Titanium Company for preparing metallic titanium or titanium alloys through high-temperature electrolytic molten titanium slags. The obtained metal titanium or titanium alloy could be directly used for ingot casting to simplify the process [49–50]. Jiao and Zhu [51–52] developed a new type of thermal reduction-electrolytic titanium extraction, namely, USTB, bearing a resemblance to the basic idea and process route of the MER process. These two approaches extract metallic titanium using soluble anode. The only difference between the two approaches is that the soluble anode of the MER process is titanium dioxide or titanium low valence oxide and that used in the USTB process is TiO·mTiC solid solution. The USTB method overcomes the low current efficiency in the FFC and OS methods. After industrial-scale achievement, the process gradually expanded from 1–10 A to 100 A scale.
At present, most methods are still in the laboratory development stage. Molten salt electrolysis may replace the existing traditional process and further be developed by shortening the process, reducing energy consumption, and realizing continuous production.
Developing new battery medium is a main stream direction in view of the excellent electrochemical performance of molten salts. High-temperature molten salt battery is a thermally activated reserve battery using molten salt as the electrolyte. As a new type of reserve battery, the internal heating agents are ignited using an automatic activation device to melt the electrolyte. It was first invented by Erb in Germany at the end of World War II. Since the first thermal battery was produced by Wurlitzer in 1948, it has received significant attention from many researchers, who then extensively studied the thermal battery from various perspectives. The thermal battery has been or will be widely applied in many fields, such as rockets, missiles, and emergency electronic instruments, because of its fast activation speed, high specific power, long storage time, high specific energy, and wide operating temperature range.
The cathode materials WO3, CaCrO4, Fe2O3, V2O5, CuO, and FeS2 are typically used in battery for in high-tech weaponry. The anode material is Mg, Ca, Li, or their alloys, and the electrolyte is high-temperature molten salt. LiCl–KCl is often used as an electrolyte for thermal battery owing to its low price and easy preparation. In addition, it is suitable for high-temperature and small current discharge with the highest peak discharge voltage and the longest discharge time [53–54]. However, Li+ is likely to form a large concentration gradient when the thermal battery is discharged rapidly, which may cause the polarization effect of the battery. Moreover, the melting point increases because of the imbalance of Li+/K+ ratio, shortening the service life of the thermal battery. Therefore, the electrolyte system has been continuously improved, and many molten salt systems, such as LiX–KX (X = Cl, Br, I), LiF–LiX (X = Cl, Br, I), LiF–LiBr–KBr/LiCl, LiCl–KCl–NaCl/LiI/KI/LiF/LiBr, and LiCl–LiI–KI/LiF/LiBr/–LiF–LiBr, have been proposed to maximize the battery life. The chloride electrolyte has a high melting point temperature; however, it has been used in thermal battery systems owing to its large electrical conductivity. With the continuous deepening and development of molten salt battery research, this technology has become increasingly mature.
In recent years, the rapid development of thermal battery technology has led to a significant interest to adapt this technology for possible select domestic applications. However, it requires the use of electrolytes with low temperature and melting points of ≤300°C. The above high-temperature electrolytes are unsuitable for this application. The incorporation of iodide ions produces a number of promising candidates, including LiBr–RbBr/CsCl/CsBr, LiCl–KCl–CsCl/–RbCl–CsCl, LiI–KI/–CsI–LiBr/–CsI–LiCl–LiBr, and LiBr–KBr–CsBr [10,55]. However, batteries based on iodides ions are heavier than those based on LiCl–KCl eutectic systems because of the higher density of iodide salts, especially for all-iodide systems. These salts are also more expensive than the bromides and chlorides. In addition, iodide electrolytes are sensitive toward oxidation forming elemental iodine, resulting in early failure of batteries. By contrast, some nitrate-based systems offer more potential because of their much lower melting points, difficult oxidation, and deliquescence. Currently proposed nitrate systems include LiNO3–KNO3, LiNO3–KNO3–CsNO3, LiNO3–NaNO3, and NaNO3–KNO3. The use of nitrate as the electrolyte is expected to become the main research direction of thermal batteries owing to its low-melting temperature and large electrical conductivity [56].
The rise of the solar thermal power system in recent years has provided a relatively inexpensive source of energy for industrial development while simultaneously reducing the consumption of ore resources and emission of pollutants. Initially, the steam from direct heating was used to drive the turbine for generating electricity. This method faces some problems, such as low production efficiency, high working pressure, and complicated system design. Subsequently, oil was proposed as the heat transfer medium for solar power generation [57–58].
Compared with heat transfer using oil and water, molten salts have higher heat stability, stronger heat transfer capacity, lower requirements for pipeline pressure, and better heat storage capacity. They can be used at higher temperatures and lower costs than the current systems and have become widely recognized as a thermal energy storage medium [59–60]. The operating temperature of solar power plants can be increased in the range of 723–773 K by using molten salt energy storage for power generation. Since the 1980s, researchers have used molten salt as a thermal transfer and storage medium in solar thermal power generation. In 1981, the Eurelios tower solar power plant was the first one constructed using molten salt as a thermal transfer and storage medium. Few decades thereafter, countries such as Spain, the United States, Denmark, Germany, and China also investigated molten salt thermal storage technology. Solar power plants use molten salt thermal storage technology to achieve flexible power production and maximize effectiveness in modern power grids.
The thermal storage media of solar power generation devices fall into three main categories: solar salt (60% NaNO3–40% KNO3), Hitec (7% NaNO3–53% KNO3–40% NaNO2), and Hitec XL (45% KNO3–48% Ca(NO3)2–7% NaNO3) [61–62]. Table 2 shows the melting points and costs of different thermal storage materials.
| Material | Melting point / K | Temperature change / K | Material cost / ($·kg−1) | Thermal storage cost / ($·kW−1·h−1·t−1) |
| Hitec | 415 | 473 | 0.93 | 10.7 |
| Hitec XL | 393 | 473 | 1.19 | 15.2 |
| solar salt | 493 | 473 | 0.49 | 5.8 |
A nuclear reactor, also known as an atomic reactor, is a device that can maintain a controllable self-sustaining fission chain reaction to convert nuclear energy into electrical energy and power. Nuclear fission releases a lot of energy and neutrons; therefore, nuclear fission energy can be advantageously applied to nuclear energy heating, nuclear power, and so on. Fission neutrons are mainly used for nuclear technology in medicine, industry, agriculture, and other aspects. Nuclear reactors mainly contain six types of reactors according to the coolants, including molten salt reactor (MSR), gas-cooled fast reactor, lead-cooled fast reactor, sodium-cooled fast reactor, supercritical water-cooled reactor, and very-high-temperature reactor. The MSR is a liquid fuel reactor in which nuclear fuel is dissolved in liquid fluoride molten salt. Compared with other reactors, the MSR holds the advantages of neutron economy, higher power density, larger negative temperature coefficient, nuclear non-proliferation, lower fuel consumption, and inherent safety. The extensive development of the atomic energy industry and nuclear fuel technology has opened new applications of molten salts. Molten salts were initially applied in nuclear reactor research in the 1950s. The Oak Ridge National Laboratory (ORNL) successfully built and operated the first MSR. The ORNL also designed a new molten salt breeder reactor with ThF4 as the fertile component of the liquid fuels and with LiF and BeF2 as the carrier salt mixtures. MSRs still have a wide room for investigation and development [6].
In China, the Shanghai Institute of Applied Physics launched a Thorium MSR Nuclear Energy System project in January 2011 as a strategic pilot project. The Chinese Academy of Science completed the design of thorium-based MSRs with solid and liquid fuels, in parallel with efforts for promoting their construction in the near future. The research of neutronics modeling, safety analysis, thermal-hydraulics modeling, and material investigation provided abundant basic data for the development of MSRs [63].
Fuel cell, also called an electrochemical generator, is a power generation device that directly converts the chemical energy of fuel into electrical energy. Fuel cells around the world have received increasing attention owing to their high-energy conversion efficiency and low environmental pollution [64].
The electrolyte in the fuel cell may be aqueous solution (e.g., H3PO4), molten salt (e.g., Li2CO3), solid polymer (e.g., polyoxyethylene), or solid oxide (e.g., Ce0.8Sm0.2O1.9). Compared with aqueous electrolytes, molten salts are ionic melts with higher conductivity. The energy conversion efficiency of the cells can reach 50%. Fuel cells are being investigated, and most of them are based on molten carbonate fuel cells (MCFCs). They are high-temperature fuel cells using molten carbonate salt mixtures as the electrolyte with normal carbonate mixtures of alkali metals Li, K, Na, and Cs. They consist of a porous nickel anode and a porous lithium-doped nickel oxide cathode[65–66]. These components are easily degraded because of the corrosive molten alkaline carbonates and the high operating temperature. The state-of-the-art cathode is usually formed by in situ oxidation and subsequent lithiation of pre-sintered porous nickel plates. In situ doping of Li provided by the Li2CO3 component of the molten carbonate electrolyte can improve the conductivity of the NiO cathode. However, the dissolution of NiO in the carbonate melt during the operation of the cell creates a problem. The characteristics of the catalyst or electrolyte should be optimized to overcome this problem. The latest MCFC anode catalyst has a satisfactory creep resistance and stable catalytic activity during fuel cell operation by using H2 or H2/CO.
The development and exploration of secondary metal resources have received considerable attention owing to the scarcity of mineral resources, the low utilization of traditional development technologies, and serious pollution. Recycling metals from secondary resources can meet the growing demand of the world metal market while achieving efficient utilization of secondary resources. The treatment methods of metal waste may differ with respect to different production industries [67–68]. Molten salt electrolysis has been applied in secondary resource recycling, such as the magnet scrap, nuclear waste, metal/alloy waste, and cemented carbide. Recycling and manufacturing of metals and alloys have undeniably become an important productive process of the present-day world [69].
Recycling rare-earth elements (REEs) from secondary resources have attracted attention with the rapid growth in the consumption of rare-earth resources and processing costs. The dependence of modern industrial manufacturing on primary products can be reduced through utilization of secondary resources. Moreover, the geographic supply distribution of REEs is changed to alleviate the resource shortage, accounting for 26.3% of the total global rare-earth consumption for rare-earth permanent magnet (REPM), which is used in electronics, such as loudspeakers, mobile phones, and hard drives [70]. At present, REPM scrap is mainly recycled through metallurgical processes. However, the available technologies and innovations need to be adjusted because of the complex design and large number of elements in the REPM scrap.
Currently, recycling REPM scrap through molten salt electrolysis has two mechanisms: (i) reacting the auxiliaries with rare-earth ions in REPMs to dissolve the REEs into the molten salts and (ii) extracting REEs by electrochemical electrolysis.
Abbasalizadeh et al. [71] used AlCl3 as a chlorinating agent. NdCl3 and DyCl3 were produced by chlorination reactions in which Nd and Dy in the magnet scrap were effectively dissolved into the molten salt phase. In addition, FeCl3 and BaCl2 were dissolved in the molten salts. Therefore, the formed molten chlorides were electrolyzed to deposit Nd and Dy at the cathode by using LiCl–NaCl–KCl mixture. This process effectively prevents the dissolution of Fe and B impurities and improves the purity of the product by changing the chlorinating agents to selectively dissolve metal ions. This method was further developed by Hua et al. [72]. The melt chlorination treatment was carried out in a dry argon atmosphere using MgCl2–KCl after pulverizing the NdFeB waste magnet into granules, as shown in Fig. 2. The overall extraction efficiency for REEs exceeds 90% under optimum operating conditions. By contrast, Fe and B remain in the solid residue. The obtained REECl3 in the molten MgCl2–KCl could be processed with molten salt electrolysis for the production of Mg–Nd alloy, realizing the efficient and selective recovery of REEs from NdFeB magnet scrap.
In addition to chlorides, AlF3, ZnF2, and FeF3 can also be added to fluoride salts as fluorinating agents to extract REEs. REEs in the NdFeB magnet scrap are more easily dissolved in fluorides than in chlorides because of the unique physicochemical properties of fluorides [73–74]. The REEs in the magnets were replaced by Al, Zn, and Fe in AlF3, ZnF2, and FeF3, respectively. The formed rare-earth fluorides could subsequently be treated by the electrolysis in the same reactor for REEs to be deposited as metal and alloy products.
Although these auxiliaries contribute to separate REEs from other elements, the reduction potentials of Mg, Al, Zn, and Fe in the auxiliaries are higher than those of rare-earth ions, resulting in the formation of the corresponding rare-earth alloys. This method raises some questions, and further separation of rare-earth alloys is necessary to produce metallic rare-earth products, making the process cumbersome.
Another way is to directly use REPM scrap as the anode without converting to halides. The REEs in the REPM electrode are selectively oxidized and dissolved in the molten salts as rare-earth ions. The electrodeposition of REEs was successfully carried out on the cathode. This method effectively avoids the problem of REEs forming alloys with impurities. Rare-earth metals have higher reactivity than iron group metals and precious metals. The thermodynamic calculation confirms the feasibility of selectively dissolving rare-earth metals from NdFeB magnet scrap [75]. Martinez et al. [76] recovered rare-earth metals from REPM scrap by using LiCl–KCl and NaCl–KCl molten salt systems. The LiCl-based melts have a larger electrochemical window than the NaCl-based electrolytes. The presence of molten salts causes a noticeable difference in the reduction potential between lithium and neodymium. Thus, molten salts are more suitable as an electrolyte.
This method is efficient and environmentally friendly while eliminating oxidation or halogenation processes; however, Li-free Nd metals are difficult to deposit because of the under potential deposition of lithium. The co-deposition of Li could be avoided by increasing the activity of REEs ions in the electrolyte and changing the electrode materials while improving product purity and electrolytic efficiency [77–78]. Konishi et al. [79–80] proposed a method to increase the activity of electrolyte ions and recover rare-earth metals in magnet scrap by using alloy membranes as cathodes. The electrolytic potentials of Dy and Nd were investigated in a molten LiCl–KCl–DyCl3–NdCl3 system. The rare-earth ions are reduced on the anolyte side of the alloy diaphragm. The rare-earth metals in the alloy diffuse onto the cathode surface of the alloy diaphragm and dissolve into the catholyte as rare-earth ions by anodic oxidation. High-purity rare-earth metals are formed on the cathode through the electrodeposition of rare-earth ions; however, the REEs in the outer layer are selectively dissolved, whereas the REEs in the inner layer remain. Kamimoto et al. [81–82] also encountered the same problem during the experiment. They performed potentiostatic electrolysis in the potential range of –1.8 to –0.8 V by using a molten eutectic mixture of LiCl–KCl. The total REE content of the electrodeposit on the liquid–zinc cathode is more than 99.2wt%. The REEs first leach from the boundary phase during dissolution because the leaching rate of REEs is slower in the main phase than in the boundary phase.
Fe oxidation occurs in the scrap owing to the relatively slower diffusion rate of REEs from the interior wastes into the outmost surfaces. As a result, the oxidized Fe products and the REEs deep into the interior wastes cannot reach the surface for the reaction. Some anode channels possibly form at the REPM electrode through oxidation to facilitate the diffusion of REEs. These channels can provide continuous transmission paths between the electrolyte and the REEs inside the electrode, thereby promoting the diffusion of REEs onto the surface of the REPM electrode. In addition, the temperature of magnet scrap electrolysis in LiCl–KCl molten salt is higher than the eutectic temperature, resulting in the volatilization of the salt.
These problems can be solved by changing the molten salt system and making some anode channels. Yang et al. [83] recovered neodymium and praseodymium from an REPM scrap through electrolysis in the LiF–CaF2 system, which does not release anode gas. Nd and Pr are selectively oxidized from the REPM scrap and transformed into rare-earth ions. The separated ions are directly prepared as rare-earth metals by electrolytic deposition, whereas the porous Fe2B alloy and Fe are left at the bottom of the crucible. The pores and channels are formed by the oxidation of REEs in Nd–Pr and Nd2Fe14B alloys through controlled current. This microstructure provides continuous transport access of the electrolyte to the inside of the REPM electrode, facilitating the oxidation of REEs inside the REPM scrap.
The post-processing of spent nuclear fuel first involves the separation of spent oxide fuel from the zirconium cladding hulls and consists of four steps: cutting, decladding, voloxidation, and exhaust gas treatment to maximize the separation of spent fuel from the spent cladding hulls. Then, the metallic elements in the waste are recovered by molten salt electrolysis.
Owing to the consumption of fissile nuclides, the formation of heavy nuclides, and radioactive fission products, reactivity changes in nuclear fuel and uranium content decreases during the reaction, producing nuclear wastes that cannot be used in nuclear reactions. This type of nuclear wastes is referred as spent nuclear fuel. Spent nuclear fuel contains a large amount of radioactive elements, such as uranium-238, thorium-232, plutonium-239, uranium-235, uranium-233, and transuranic elements, which are harmful to the environment and human health. Appropriate countermeasures must be taken to avoid their serious impact. As early as the 1980s, the pilot-scale electric refiner (Mk-IV ER) was used in the development and research of electrorefining technology for spent fuel by the Argonne National Laboratory in molten LiCl–KCl/liquid cadmium systems. Uranium elements were separated from the fission products to reduce radioactive waste emissions. Afterward, the United States launched an engineering research and development of molten salt electrolytic extraction to recover plutonium and minor actinides [84–85].
The electrolysis of the spent fuel usually involves two main recovery steps. First, the alkaline metals and alkaline earth metals in the spent fuel are dissolved into molten salts by molten salt electrolytic reduction, and the actinides and fission products are separated by molten salt electrolytic refining.
Spent fuel is used as the cathode and molten Li2O–LiCl as the electrolyte in electrolytic reduction. Actinide metal oxides are reduced to metallic elements and deposited on the cathode during electrolysis following Eqs. (1) and (2):
|
Cathodereaction:MxOy+2ye−→xM+yO2−(salts). |
(1) |
Oxygen ions (O2−) generated at the cathode are oxidized to O2 through O2− diffusion to the anode:
|
Anodereaction:2O2−(salts)→O2(g)+4e−. |
(2) |
The dissolution of O2− ions exuding out to the surface from the inside of the oxide fuel into the molten salts is difficult and obviously affects the reduction rate and current efficiency during electrolysis. Consequently, Li2O is added to the molten LiCl to accelerate the electrochemical reaction. By contrast, Li2O is separated by electrolysis, depositing metallic lithium on the cathode. The metallic lithium can penetrate into the cathode for reducing oxides to metals, simultaneously oxidizing Li to Li2O again, realizing the self-circulation of Li2O [86].
Achieving high current efficiency of electrolytic fuel is impossible because of the large and dense nature of the original spent oxide fuel. Therefore, the uranium in the spent fuel is proposed to convert to U3O8 powders after oxidation at ~773 K for improving the current efficiency. Hur et al. [87] studied a new method of treating U3O8 in the spent nuclear fuel by using an SUS conductor and porous magnesium oxide film as the cathode in Li2O–LiCl molten salt. In addition, U3O8 powders were used for simulation test to clarify the reaction mechanism and verify the feasibility of the method. The conversion rate of U3O8 to U metal exceeds 99% after constant current electrolysis, proving that the electrochemical reduction technology is suitable for the treatment of spent oxide nuclear fuel. In addition, uranium metals are successfully deposited in LiCl–Li2O molten salt [88]. This cathode assembly successfully achieves a scale-up experiment of reducing U3O8 to metallic uranium by molten salt electrolysis in LiCl–Li2O molten salt. Within the potential range of −2.47 to −3.46 V, the conversion rate of U3O8 to metallic uranium exceeds 99% in a 20-kg U3O8 batch cell [89].
Although the obtained uranium metals were successfully reduced by U3O8 through electrochemical reduction, the application of this process is still limited by the cumbersome preparation of U3O8, excessively thick cathode basket, and decreased Li2O concentration in the electrolyte after electrolysis.
Subsequently, many researchers converted uranium elements in the spent fuel into porous UO2 particles for electrochemical reduction. Compared with U3O8, porous UO2 particles are easier to handle because of the bulk structure and less loss of Li2O in molten salts. Sakamura and Omori [90] fabricated UO2 with a porosity in the range of 30%–38% by sintering U3O8 powders and performed electrolysis in LiCl–Li2O electrolyte at 923 K for 10 h. Experimental results showed that metal uranium could be produced from UO2, achieving electrolytic reduction step with a degree of efficiency exceeding 62%. The reduced product was then loaded into the anode basket, and the electrorefining step proceeded in the LiCl–KCl–UCl3 electrolyte at 773 K. The anode current efficiency can reach 88%, and dendritic refined uranium metals are deposited on the stainless steel cathode.
The experimental observation proved that simulating the porous structure of spent fuel particles accelerates electrochemical reduction. However, the low porosity of the prepared UO2 decreases the electrolysis efficiency. Choi et al. [91] found that the electrochemical electrolysis proceeds at −0.49 V in LiCl–Li2O medium at 923 K by using the prepared porous simulated spent fuel particles (porosity of 70.7%) as the anode. Fig. 3 shows the experimental setup for the electrochemical reduction.
Analysis results showed that the reduction extent of UO2 is >99%. A large amount of rare-earth oxides are reduced to the corresponding metals in the range of 46%–78% because of the porous structure of the particles. The experiments demonstrated that the highly porous structure of spent fuel particles is conducive to accelerating electrochemical reduction. Choi et al. [92] also experimentally investigated the effect of the cathode/anode surface area ratio on the electrochemical reduction of uranium oxide in the Li2O–LiCl electrolyte, indicating that a low cathode/anode surface area ratio increases the current density. A 17-kilogram uranium oxide electrolysis cell was set up to reduce uranium oxide to metallic uranium at a cathode/anode surface area ratio of 2.6.
A new problem may arise from the disposal of radioactive molten salt wastes after the electrochemical reduction process. Lithium has been recovered from molten salt waste consisting of LiCl, Li2O, Cs2O, and SrO [93] by conducting electrolytic reduction experiments in a single compartment electrochemical reactor with a mono-polar connection. The cesium and strontium oxides in the mixed salts could be converted to chlorides by controlled LiCl concentration and applied potential. In addition, Li2O preferentially reduces to metallic lithium in the potential range of 2.55–3.55 V.
For the sake of separating actinides and fission products, at first, the obtained pure uranium metals are separated from the fission products. Next, transuranic elements and fission products are separated for plutonium, uranium, and minor actinides. The principle of electrorefining is to separate the various metallic elements based on the differences in the thermodynamic and electrochemical properties of the involved elements in molten salts. The chopped scrap metal fuel is placed into the anode basket, or the reduced metal obtained at the cathode after electrolytic reduction is used as the anode. The rare-earth fission elements are replaced with the pre-dissolved UCl3, and the generated chlorides are dissolved in the LiCl–KCl eutectic salts. Uranium is dissolved from the anode into the molten salts and eventually deposited on the cathode during electrolysis. As a result, insoluble zirconium and other rare-earth metals remain in the anode basket, while 99.7% of actinides in the spent anode fuel are dissolved in the molten salts. Most of the dissolved uranium is deposited on the solid cathode, forming a dendritic structure. The minor actinides and uranium elements are electrodeposited on liquid metal cathodes (liquid Cd or Bi) [94].
However, liquid cathodes suffer in terms of low separation coefficients of transuranium and other metallic elements, posing difficulty in separating cadmium from the products and volatility of liquid cadmium under high temperatures. Solid cathodes are expected to overcome the disadvantages of liquid cadmium cathodes. Kinoshita et al. [95] studied the electrodeposition of uranium and transuranium elements on solid cathodes and compared the effects of liquid and solid cathodes on extraction. Kwon et al. [96] investigated the electrochemical separation of actinides in LiCl–KCl eutectic salts by using a perforated ceramic container tied with a molybdenum rod as solid cathode. The anode potential decreases with increasing anode surface area. The separation of uranium and cerium is achieved after electrolysis.
However, dendritic deposits are produced when using solid metal electrodes, making the products difficult to separate from the solidified salt after electrolysis because of entraining the molten salt medium. In addition, the current efficiency of the molten salt is lower than that of the liquid electrode. The deposits on the solid cathode are easy to fall into the molten salt from the electrode. Thus, most of the process still retains the liquid cathode.
At present, the fuel rods of the pressurized water reactor type nuclear power plant and the surrounding cladding hulls are designed with Zr-4 alloy as the main raw material. The corrosion of Zr-4 alloy can be easily accelerated in the high-temperature and high-radiation reaction environment. This process requires regular disassembly and replacement. Spent cladding hulls should contain ~98% of Zr and other metal elements (Sn, Fe, and Cr) and a large number of radioactive elements and trace amounts of spent fuel after a long-term reaction. Reasonable means of recycling spent cladding hulls not only benefit the secondary recycling of resources but also are an irreplaceable tool for environmental protection.
The most representative recovery method is molten salt electrolysis, recovering high-purity Zr from the spent cladding hulls by applying an appropriate potential/current [97]. The reactions occurring at anode and cathode are as follows.
|
Anodereaction:Zr→Zrn++ne− |
(3) |
|
Cathodereaction:Zrn++ne−→Zr |
(4) |
Using various electrolyte components, many investigators studied the effects of molten salts containing ZrCl4 or ZrF4 on the electrolysis efficiency and Zr metal electrodeposition. Lee et al. [98] explored the recovery of Zr from spent Zr-4 cladding hulls in LiCl–KCl–ZrCl4 molten salt at 500°C. Under the same experimental conditions, the identical experiment was conducted with the spent Zr-4 cladding hulls as the anode to verify the effectiveness of Zr electrolysis. Quantitative analysis revealed that the purity of the recovered Zr deposits is 99.44wt%.
However, the reaction rate is relatively slower compared with chlorination, making large-scale spent cladding hulls difficult to dispose. Oxidation reactions also occur in the cladding hulls during actual electrolysis, resulting in the coating of the surface of cladding hulls with non-conductive Zr oxides. The obtained oxides significantly inhibit the reduction/oxidation of Zr. For specimens with thin oxide layers, further research showed that Zr oxides could be effectively dissolved by pretreatment and adjustment of the anode potential, temperature, and sufficient reaction time. Surface and cross-sectional analyses of the cladding confirmed that the electrochemical dissolution of the Zr oxide film is not uniform under a short period of time; however, Zr oxide film disappears after a long period of electrochemical dissolution at a low temperature. The simple dipping of LiCl–KCl–ZrCl4 molten salts changes the surface composition from a high-valent oxidation state to a low-valent oxidation state, forming Zr suboxides such as Zr2O3 and ZrO. For the thick oxide layer covered with more than 10 μm, the effect of molten salt pretreatment is not obvious and an electrical contact through cutting edges is necessary to promote Zr dissolution [99–101].
LiF is added to improve the electrolysis efficiency and stabilize the valence change of Zr in the molten salt, thereby avoiding disproportionation. Fujita et al. [102] verified the feasibility of the recovered actual spent Zr cladding hulls through two-stage electrorefining in the LiCl–KCl–LiF system. The content of radioactive elements decreases after electrolysis, and the purity of the recovered Zr deposits is 99.9wt%. The redox potentials of radioactive elements Co-60, Sb-125, Nb-95, and Mo-93 in the actual spent cladding hulls are higher than the positive charge of Zr. Thus, the radioactive elements are deposited on the bottom of the anode basket by applying different anode voltages in the LiCl–KCl–LiF system. The radioactivity of the purified Zr sample is significantly reduced. The Zr ions in the molten salts are recovered at the cathode by applying a cathode voltage after removing radioactive impurities, enabling a new decontamination process for a spent zircaloy [103]. The addition of fluorides enhances the formation of stable complexes, eliminating the multi-step reduction process and improving current efficiency. By contrast, all fluoride-based salts enable the formation of a dendritic Zr deposit and enhance the crystallinity of Zr. As far as the suitability of the process for treating waste is concerned, the fluoride-chloride molten salt system has advantages over the all chloride-based or all fluoride-based molten salt system.
For Zr deposition, chlorides have been widely used because of their lower operating temperatures and less corrosive nature than fluoride-based salts. However, the powder-type product characteristics of Zr in most chloride-based molten salts decrease the recovery efficiency because of easy oxidation. In fluoride molten salt, the formation of dendritic Zr deposits may be caused by the single-step reduction of Zr4+ [104]. Nevertheless, processes using fluoride salt require several challenges for the waste treatment because of its high melting point and strong corrosivity. This technology is currently in the developing stage and should be explored in the near future for the application of all-fluoride salts.
The high-level radioactive waste liquid from nuclear power plants is mainly processed using evaporation and chemical precipitation and can be treated through molten salt electrolysis to obtain clean products, thereby minimizing damage to the environment and bringing economic benefits. Separation methods for transuranium elements from high-level radioactive liquid waste had been studied [105]. Initially, oxalic acid is added to the high-level radioactive waste liquid to precipitate transuranium elements, lanthanides, and alkaline earth metals for removing soluble alkali metals and precious metals. The precipitation method can recover 99.9% of transuranium elements from the liquid waste and then convert oxalates to chlorides. The transuranium elements are deposited on the cathode by molten salt electrolysis after these chlorides dissolve in KCl–LiCl salt. Compared with the traditional methods, this partitioning process has lower usage amount of corrosive gas and less energy consumption, achieving good separation of transuranium elements from liquid waste.
Aluminum slags are the byproducts of aluminum smelting or remelting. Each year aluminum output of the aluminum plant is 1 t, which consequently leads to 10 kg of slags. Worldwide, approximately 250000 t of slags containing 75wt% Al are generated every year with high development value. Therefore, the extraction of aluminum from slags facilitates the green and effective applications of secondary resources and will be potentially beneficial for the aluminum industry in the long run. The current thermal reduction for aluminum slags is relatively efficient and energy saving; however, further purification treatment is required for low-purity products. Molten salt electrolysis can produce pure products with comparatively low energy consumption [106]. Ueda et al. [107–108] conducted the flotation of aluminum alloy oxides and electrolysis of metallic aluminum in two molten salt systems, AlF3–NaF–BaCl2 and NaCl–NaF–BaCl2. At first, 83% of the aluminum alloy is recovered by the flotation from A356.0 casting alloy slags in the molten salt bath because of the difference in density between the aluminum alloys and the oxides. Thereafter, another 4% of metallic aluminum is recovered from the molten salts after flotation by electrolysis.
The aluminum slags contain a large amount of AlN. Thus, a significant amount of work is required to recover Al from aluminum slags with 30wt% AlN. Consequently, Yan [109] investigated the recovery of aluminum in aluminum dross (containing metallic Al, Al2O3, and AlN) by combining calcium thermal reduction and molten salt electrolysis in Ca–CaCl2 molten salt. Four simulated systems, including pure Al2O3, pure AlN, Al2O3/AlN mixture, and Al2O3/AlN/Al mixture, were used to simulate the reduction of aluminum slags to understand the reduction behavior. The studies showed that Al2O3 is chemically reduced by Ca to form Al-rich Al-Ca alloys. The Al in the Al2O3 is easily recovered from the Al drosses, while AlN cannot be reduced. The reduction of AlN could be enhanced by adding Al2O3 to AlN. In addition, the direct electrochemical reduction of the AlN in the drosses is limited to three phase boundaries among the AlN, the electrolyte, and the current collector. The presence of Al powders in the Al2O3/AlN mixture also effectively facilitates the direct electrochemical reduction of Al2O3 and AlN. During electrolytic reduction, aluminum can be recovered from slags containing Al2O3; however, directly extracting aluminum from AlN slags is difficult. Therefore, the AlN in the aluminum slag could be indirectly converted into Al2O3 to extract Al.
Zr scrap mainly includes industrial wastes generated in the manufacture of corrosion-resistant mechanical, steel, and other alloy products; unqualified Zr sponges produced in the Kroll process; and slags produced in the metallurgical process. The major methods of recycling Zr from Zr scrap include combined chlorination, chemical separation, and Kroll process. However, several difficulties still persist because of low recovery rate and high cost with the conversion rate between 25% and 40% from sponge zirconium to zirconium alloy. The noticeable advantage is that high-purity Zr or even nuclear-grade Zr metal can be directly obtained from Zr scrap by molten salt electrolysis [110]. The scrap is converted into dendritic zirconium crystals in NaCl–K2ZrF6 or NaCl–NaF–ZrCl4 during molten salt electrolysis. The obtained products meet reactor-grade specifications with high purity than the traditional process. In addition, electrolysis is more economical and environmental friendly than the chlorination-Kroll reduction of Zr scrap.
However, the Zr deposits generated in the simple electrochemical recovery are powders easily exposing metal surfaces to oxidation because of the increase in the surface area during the reaction. At the same time, the low current efficiency from the disproportionation reaction of Zr results in low product purity and unstable reaction. Accordingly, the morphology and purity of Zr deposits in fluoride electrolytes could be controlled by varying current densities. The aim of addition of ZrF4 is to effectively curb the disproportionation reactions between Zr and ZrF4, leading to a stable Zr4+/Zr reduction process in the fluoride electrolytes. The advantage of this simple method is that the high-purity nuclear-grade dense Zr deposits are directly recovered from Zr scrap [111].
Park et al. [112] carried out the fluoride salt of LiF–KF–ZrF4 by using multiple electrodes for large-scale production and improving throughput Zr electrorefinery development to improve the cell efficiency and suppress the disproportionation reaction. Experimental results showed that the amount of applied current increases because of decreasing cell resistance with increasing number of cathodes, thereby improving the current efficiency compared with a single electrode. Under optimum conditions, the recovery rate of pure Zr reaches 99.92% with impurity content lower than that using the conventional anodes (97.8%). Electrorefining consumes approximately 7.15 kW·h·kg−1, less than 39.7% compared with the Kroll process. The cell efficiency and metal recovery rate are significantly improved, providing an important experimental basis for scale-up electrorefining Zr scrap using multiple cathodes.
Disposal of titanium blast furnace slags from alloy steelmaking is confronted with the problems of difficult metal extraction, long cycle time of the reaction process, and complicated procedure. Toxic metals such as chromium and vanadium in the slags also leach out simultaneously. At present, blast furnace slags are mainly piled up in the slag yard, causing great pollution on the surrounding environment. Therefore, finding a more reasonable and effective way of utilizing the slags is urgently required.
Zou et al. [113–115] first proposed to prepare titanium-silicon alloys, including Ti5Si3, Ti5Si3/TiC, and Ti5Si3/Ti3SiC2, through molten salt electrolysis from titanium-containing blast furnace slags. Ti5Si3 was synthesized in molten CaCl2 electrolyte with pre-fired titanium-containing blast furnace slag/TiO2/SiO2 (Ti : Si = 5:3) as a cathode. The optimal electrolysis conditions, ultimate product morphology, and phase composition were studied. Experimental results indicated that Ti5Si3 alloy is the product of electrolytic reduction and effectively removes impurities, such as calcium, magnesium, and aluminum, from the titanium blast furnace slags. Fig. 4 illustrates the schematic of the electrolytic cell and the experimental setup of the process.
Nevertheless, graphite-based anode electroreduction for preparing alloy suffers in terms of low reaction rate, low efficiency, and carbon contamination. Therefore, pressed porous mixture pellets were proposed as the cathode and a solid oxide oxygen-ion-conducting membrane as the anode. TiSi, TiSi/TiC, and TiSi/TiSiC had been electrochemically synthesized from the Ti-bearing blast furnace slag/TiO2 and/or C mixture precursors in molten CaCl2 [116]. The electrical reduction of titanium blast furnace slag/TiO2 and C mixture precursors includes the initial decomposition of Ca(Mg,Al)(Si,Al)2O6, the reduction of Ti/Si-containing intermediate phases, the removal of impurity elements, and the formation of Ti5Si3, TiC, and Ti3SiC2. This inventive method has great potential to be used for the direct and facile preparation of titanium alloys and titanium-based composite materials. In particular, the SOM-based anode increases reaction speed and effectively avoids carbon contamination compared with the consumable graphite-based anode electroreduction.
Cemented carbide scrap melts at a much higher temperature, making its recycling more difficult than other metal wastes owing to its high strength, hardness, and density. Therefore, the recycling methods of cemented carbide scrap are discussed separately.
The main component of cemented carbide is tungsten carbide (WC) with a hardness close to that of diamond. These WC phases are combined with tough binder metals (most commonly cobalt, nickel, and molybdenum) to form cemented carbides by powder metallurgy for specific industrial applications. Cemented carbide, typically containing 74%–91% tungsten, is superior to that of tungsten ores (7wt%–60wt% tungsten). Therefore, the recovery of tungsten from cemented carbide scrap is worthy considering owing to its considerably higher tungsten content compared with primary ores. As the concept of sustainable development gains ever-increasing importance in the future, efficient and economical recovery and re-use of the finite resources of tungsten can be achieved from cemented carbide scrap [117–120].
At present, the recycling methods of cemented carbide scrap are mainly divided into two categories. One is selectively extracting cobalt retaining the structure of WC through acid leaching, zinc melting, and high-temperature treatment. The performance of the generated products is generally worse than the raw ore because of the lack of atomic regeneration. In another embodiment, complete smelting destroys the structure of the alloy. However, further development has so far been constrained by long recovery time and high cost. Molten salt electrolysis has been proven simple, environmentally friendly, and low investment with a bright application prospect. Several studies used molten salt electrolysis to extract tungsten from various tungsten-bearing compounds, such as tungsten oxide, tungsten chloride, tungsten sulfide, and tungstate. However, few studies focused on recovering tungsten from cemented carbide scrap [121–124].
Xi et al. [125] first presented a new technology of electrochemical extraction of tungsten and cobalt from cemented carbide scrap by molten salt electrolysis. WC and NaCl–KCl salt were used as the consumable anode and the electrolyte, respectively. Fig. 5 illustrates the molten salt electrolysis of cemented carbide scrap. The optimum process conditions were obtained by analyzing the composition, particle size, and morphology of the cathode products. Different electrochemical methods were developed to explore the redox behavior of tungsten ions derived from the anode, the nucleation mode of tungsten deposition at the cathode, the number of electron transferred during the reaction, the electrochemical reaction resistance, and the electrochemical reversibility. The feasibility of the electrolysis of WC in CaCl2–NaCl, LiCl–KCl, and other chloride molten salt systems was also investigated.
The anodic dissolution of WC in molten salt can follow two pathways. (i) Tungsten in WC exists in the molten salt medium as ions and is deposited on the cathode through an electrochemical method. (ii) WC is continuously broken into particles in the molten salt during electrolysis to form ions through chlorination and finally electrodeposited on the cathode. Comparison of the anode WC phase before and after electrolysis indicated that the anode phase does not show any apparent variation and pure WC phases are observed. Therefore, WC is not chlorinated by molten salt in WC electrolysis. As the electrochemical dissolution proceeds, the part of the electrode in contact with the molten salt always remains as WC phase although the WC surface is being continuously regenerated. Cyclic voltammetry tests were carried out on the molten salt before and after electrolysis to determine whether or not tungsten ions are dissolved in the molten salt. Experimental results revealed that the tungsten in the WC is dissolved in the molten salt as ions, as confirmed by the corresponding redox peaks after electrolysis. The absence of redox peaks proves that no electrochemical reaction occurs before electrolysis. The experiments ruled out the possibility that WC was broken into particles and fell into the molten salt during electrolysis.
Although the electrolysis of WC in the chloride molten salt system shows some promising results, some problems remain. First, the anode dissolution amount and dissolution rate increase significantly with increasing temperature, considering that the reaction of WC is exothermic and the Gibbs free energy of the reaction decreases with increasing temperature during electrolysis. Although high temperature accelerates the electrochemical reaction and the dissolution of WC, high volatility of molten chloride salt still poses some problems. In addition, electrolysis processing demands considerable energy input to maintain a high electrolysis temperature. Second, the lack of discharge ions in the molten salt results in difficult polarization reaction and low current efficiency in the early stage of electrolysis. Third, the anode products not only contain tungsten ions but also elemental carbon, as observed in blanketing the surrounding anode on the molten salt surface with layers of fine black powder after cooling. In long-term electrolysis, the generated carbon is excessively accumulated. Carbon spreads in the area nearby the cathode and directly affects the purity of the tungsten powders, even causing a short circuit in the electrolytic cell.
To achieve rapid dissolution of cemented carbide and selective preparation of pure metals, our group [126–135] improved the extraction efficiency of tungsten by changing the molten salt systems and adding active substances. The characteristics of fluoride molten salt systems include low energy consumption, higher electrolysis temperature than chloride molten salt, and low volatility. Consequently, the effects of electrolysis time, temperature, current density, and other conditions for the dissolution of WC were studied in a NaF–KF all-fluoride molten salt system. The dissolution process and mechanism of WC were proposed on the basis of optimum process parameters. Results showed that NaF–KF has a relatively wide electrochemical window than NaCl–KCl. Tungsten ions might form fluoro-tungsten complex ions in fluoride molten salts to facilitate the deposition of tungsten ions at the cathode. Fluoride molten salts have the advantages of low volatility and wide temperature range, and thus can further increase the reaction temperature and accelerate the chemical reaction.
The active particles can be discharged at the cathode and anode by adding a certain amount of active substances to the molten salts. The suitable active substances acting as a carrier of tungsten ions can improve the dissolution rate of the anode and the current efficiency because the addition of sodium tungstate and tungsten oxide would reduce the charge transfer resistance in the electrolysis systems. Among them, sodium tungstate is added to the chloride molten salt system, and it can provide tungsten ions with similar properties to the WC dissolved ions as an active substance, thereby improving the dissolution rate of WC and current efficiency. Conversely, the tungstate ions formed by the ionization of sodium tungstate generate oxygen at the anode during electrolysis. The generated oxygen can react with residual carbon to produce gaseous carbon oxides for eliminating the impact of carbon on cathode products and improving the purity of tungsten products. Studies on the electrolysis of WC in different molten salts are summarized in Table 3.
| Melt | Anode reaction | Cathode reaction | Overall reaction | Reduction pathway | Temperature / K |
| NaCl–KCl | WC → W6+ + C + 6e− | W6++6e−→W | WC→W+C | W6+→W4+→W | 1023 |
| NaCl–KCl– Na2WO4 | 3WC+Na2WO4→ 4W6++3CO+Na2O+24e− | W6++6e−→W | 3WC+Na2WO4→4W+3CO+ Na2O | W6+→W | 1023 |
| NaCl–KCl–WO3 | 3WC+WO3→4W6++3CO+24e− | W6++6e−→W | 3WC+WO3→4W+3CO | W6+→W | 1023 |
| NaF–KF | WC−2e−→W2++C | W2++2e−→W | WC→W+C | W2+→W | 1073 |
Cemented carbide is a composite material comprising WC embedded in different contents of Co binder. Therefore, the effect of cobalt on the recovery of tungsten from cemented carbide scrap should not be overlooked. WC–6wt%, 10wt%, and 15wt% Co cemented carbide scraps were used as the anode to prepare tungsten and cobalt powders in NaCl–KCl and NaF–KF molten salt systems, respectively. The change rules of anodic dissolution and products were obtained by electrochemical analysis and testing. A novel process for the selective recovery of tungsten and cobalt elements by the dual-electrode method and the stepwise electrolytic method was proposed based on the optimal reaction conditions, with WC–Co as the anode and two stainless steel rods as the cathode. The proposed process generates the product with high purity, excellent performance, and simple composition, thus realizing high-value recycling of cemented carbide scrap. Physical quantities such as electric field distribution, material concentration change, and electrode dissolution state cannot be directly observed by experiments because of limited experimental conditions. Consequently, simulation and analysis of the molten salt electrolysis process of cemented carbide were carried out on the basis of the experimental data. The expected data were obtained, and the number of experiments decreased by simulating the reaction process and results.
The invented molten salt electrolysis method can significantly shorten the recycling process of cemented carbide scrap. The simple and environment-friendly operation process successfully realizes the regeneration of different metal components in the cemented carbide scrap. The recovery of cemented carbide scrap should be expedited for industrialization in the near future. In the meantime, systematic investigation should be conducted to improve the current efficiency, material source, efficient separation, and regeneration of various valuable metal elements in the cemented carbide scrap. Such investigation would enrich and comprehensively describe the correlation analysis on electrolysis in the recycling process design of secondary resources.
Molten salts exhibit the advantages of high heat capacity, excellent thermal stability, and low vapor pressure and have been widely applied in various industrial sectors, such as nuclear energy, fuel cells, and metallurgical industries. The water-free and oxygen-free molten salt electrolysis conditions also provide an excellent working environment for metal processing. Therefore, further extension and development of this method for the recovery of metallic elements in secondary resources are of great significance from the economical and environmental perspectives. Even though the sustainable development of molten salt electrolysis faces several challenges in terms of electrolytes, electrodes, and electrolytic cells, it may provide considerable opportunities in the near future. The major conclusions and prospects of this study are as follows.
(1) Alkali metals and alkaline earth-metal halide salts have been commonly used as molten salt electrolytes because of their high decomposition potentials, providing a relatively small overpotential generated by cathodic polarization during cathodic deposition. At present, chlorides, fluorides, and chloride–fluoride mixtures are widely used in molten salt electrolysis. A wide range of electrodes and container materials can be used to fulfill the demand of the low operating temperature for molten chloride salts. As to disadvantages, the high volatility of halide molten salts, especially chlorides, causes fuming and loss of electrolyte. In addition, chlorides possess certain hygroscopic characteristics, react with moisture, or even decompose with water. The simple heating process cannot remove the water of hydration from the metal chlorides, complicating the electrolytic reaction. Fluoride molten salts are less reactive toward moisture and can dissolve oxides directly, thereby eliminating fluorination. They also have a wider operating temperature range but greater corrosion rate than chlorides. Reliable reference electrodes should be investigated for fluoride molten salts because the accuracy and reliability of data obtained with uncertain electrodes or quasi-reference electrodes are difficult to ensure. Another disadvantage is the relatively low solubility of solid fluorides in aqueous solution, which complicates the disposal of waste solidified fluorides after electrolysis.
Fluorides and chlorides have different degrees of corrosivity and volatility in a high-temperature working environment, necessitating the use of expensive fire-resistant and corrosion-resistant materials to seal the container and making the overall process relatively difficult. During electrolysis, constant thermal energy must be provided to maintain the molten state for a long time because of the high melting point of molten salts. In addition, inert atmospheres are required during electrolysis to ensure a water/oxygen-free environment. These factors increase the operating cost of the process. Many hygroscopic salts used as solutes and solvents must be purified to remove the water for large-scale industrial production. Purification is often a prolonged process, which necessitates considerable peripheral handling facilities and increases the operating cost of industrial production.
In-depth studies should focus on the thermal and chemical corrosion properties of molten salts, the basic theory of thermodynamics and kinetics, and the electrochemical reaction mechanism in secondary resource electrolysis. On the basis of existing research, molten salts should be explored further to fit for high-purity and high-performance electrolytic metallurgy. A new low-melting molten salt system applicable to energy-saving and environmental-friendly metal electrodeposition is required. Therefore, it is of great significance for improving the efficiency and stability of the electrolytic systems and solving the problems of high-energy consumptions, excessive emissions, and high costs of large-scale industrial production. Research and development of directly using partially dehydrated halides or non-dehydrated halides as electrolytic raw materials is also an important area for investigation.
(2) The choice of electrodes for molten salt electrolysis mainly depends on the chemical reactivity of the electrode to the electrolyte and the manufacturing cost of the electrode. The cathodes of the electrolytic cell are currently mainly divided into two categories: solid metal and liquid metal electrodes. When the metals are deposited on the solid metal electrode, the dendritic deposits entrained with molten salts are generated during the cathode reaction, complicating the separation of the dendritic metal products and the solidified salts. Therefore, subsequent treatment is inevitable for the removal of salts after electrolysis.
The liquid metal electrode effectively solves this problem and the products can be directly extracted from the liquid electrode through siphoning. However, in the cadmium liquid electrode, for example, the separation factor of uranium element is lower than those of other metals, and some target metals are difficult to separate from the electrode. The requirement of high temperatures and restricted environment is a disadvantage that needs to overcome. In the case of cadmium, considerable problems are associated with its volatilization and electrode failure.
To some degree, the development of molten salts is limited because they are inorganic materials with high-temperature resistance. Whether it is a cathode or an anode, ensuring certain corrosion resistance and high-temperature resistance is necessary. Stable current efficiency, the reactivity of target product with the electrode, and avoiding the production of greenhouse gases should be assured. Therefore, the development of suitable electrode materials is also a major challenge.
(3) The products of molten salt electrolysis are of higher purity. The process is more environmentally friendly and clean with the traditional process, whereas the output of unit volume and productivity is far below the theoretical value. The decline is mainly due to large anode–cathode distances compared with the traditional reactors, increasing diffusion distance, poor mass transfer, excessive voltage drops, and energy losses. The mass transfer in the anode cell can be enhanced by arranging two electrodes, reducing the anode–cathode distances, and stirring the liquid metal with a ceramic impeller. Moreover, the anode current density can be reduced to balance the diffusion melt in the metal cell. Therefore, effective ways of achieving high-value utilization of energy are through the development of new high-efficiency electrolysis equipment, the reasonable optimization of electrolysis structures and parameters, and the exploration of energy-saving, clean, and automated electrolysis equipment.
(4) Research on the treatment of wastes generated from molten salts is still in its infancy, and their engineering application requires considerable work. In addition, a large amount of waste residues is generated irrespective of the molten salt electrolysis technology or refining technology, requiring waste treatment prior to safe disposal. Recycling and utilizing wastes is an excellent way of decreasing the input of fresh molten salts. It can also take advantage of the solidification of molten salts for certain components in the wastes to recover high-economic value elements. However, few studies focused on the treatment of waste molten salt, requiring further exploration.
In conclusion, the current sustainable research and development of molten salt electrolysis should concentrate on the existing process operations and the design of electrolytic cells, such as electrodes, electrolytes, and cell materials, to ultimately reduce energy consumption, production costs, and greenhouse gas emissions. The personnel in the metal production industry should focus on technology and equipment improvements to avoid the high investment costs and high reform risks. The metal industries would be reluctant to make major changes. Even so, technological adjustments and mode changes are still required for the development of molten salt electrolysis and the realization of sustainable metallurgical processes. The comprehensive utilization of secondary resources must be strengthened under the current global resource shortage and increased production costs. A high-efficiency molten salt electrolysis technology must be developed with short and continuous process, low cost, and environmental protection. As matters stand, the technology of molten salt electrolytic treatment from secondary resources is still at the laboratory stage, and considerable efforts must be exerted before its application in engineering. The electrolytes, electrodes, and reactors have a great room for improvement in the near future.
This work was financially supported by the National Natural Science Foundation of China (No. 51621003) and the Beijing Natural Science Foundation (No. 2204073).
|
D.R. Sadoway, New opportunities for metals extraction and waste treatment by electrochemical processing in molten salts, J. Mater. Res., 10(1995), No. 3, p. 487. DOI: 10.1557/JMR.1995.0487
|
|
S.Q, Jiao, H.D. Jiao, WL. Song, M.Y. Wang, and J.G. Tu, A review on liquid metals as cathodes for molten salt/oxide electrolysis, Int. J. Miner. Metall. Mater., 27(2020), No. 12, p. 1588. DOI: 10.1007/s12613-020-1971-x
|
|
S.Y. Liu, Y.L. Zhen, X.B. He, L.J. Wang, and K.C. Chou, Recovery and separation of Fe and Mn from simulated chlorinated vanadium slag by molten salt electrolysis, Int. J. Miner. Metall. Mater., 27(2020), No. 12, p. 1678. DOI: 10.1007/s12613-020-2140-y
|
|
L. Kartal, M.B. Daryal, G.K. Şireli, and S. Timur, One-step electrochemical reduction of stibnite concentrate in molten borax, Int. J. Miner. Metall. Mater., 26(2019), No. 10, p. 1258. DOI: 10.1007/s12613-019-1867-9
|
|
Y.K. Wu, S. Chen, and L.J. Wang, Electrochemistry of Hf (IV) in NaCl–KCl–NaF–K2HfF6 molten salts, Int. J. Miner. Metall. Mater., 27(2020), No. 12, p. 1644. DOI: 10.1007/s12613-020-2083-3
|
|
V.M.B. Nunes, C.S. Queirós, M.J.V. Lourenço, F.J.V. Santos, and C.A. Nieto, Molten salts as engineering fluids-A review: Part I. Molten alkali nitrates, Appl. Energ., 183(2016), p. 603. DOI: 10.1016/j.apenergy.2016.09.003
|
|
R. Serrano-López, J. Fradera, and S. Cuesta-López, Molten salts database for energy applications, Chem. Eng. Process., 73(2013), p. 87. DOI: 10.1016/j.cep.2013.07.008
|
|
B. Muñoz-Sánchez, J. Nieto-Maestre, I. Iparraguirre-Torres, A. García-Romero, and J.M. Sala-Lizarraga, Molten salt-based nanofluids as efficient heat transfer and storage materials at high temperatures. An overview of the literature, Renewable Sustainable Energy Rev., 82(2018), p. 3924. DOI: 10.1016/j.rser.2017.10.080
|
|
P. Liu, Y.X. Tong, and Q.Q. Yang, Molten salt systems and the new developments for the application of molten salts, Electrochemistry, 134(2007), No. 4, p. 351.
|
|
M. Patrick and A.G. Ronald, Thermal activated (thermal) battery technology: Part Ⅱ. Molten salt electrolytes, J. Power Sources, 164(2007), No. 1, p. 397. DOI: 10.1016/j.jpowsour.2006.10.080
|
|
M. Liu, N.H. Steven, B. Stuart, M. Belusko, R. Jacob, G. Will, W. Saman, and F. Bruno, Review on concentrating solar power plants and new developments in high temperature thermal energy storage technologies, Renewable Sustainable Energy Rev., 53(2016), p. 1411. DOI: 10.1016/j.rser.2015.09.026
|
|
K. Habib, S.T. Hansdóttir, and H. Habib, Critical metals for electromobility: Global demand scenarios for passenger vehicles, 2015-2050, Resour. Conserv. Recycl., 154(2020), art. No. 104603. DOI: 10.1016/j.resconrec.2019.104603
|
|
S.E. Zhang, Y.J. Ding, B. Liu, and C.C. Chang, Supply and demand of some critical metals and present status of their recycling in WEEE, Waste Manage., 65(2017), p. 113. DOI: 10.1016/j.wasman.2017.04.003
|
|
S. Langkau, E. Tercero, and A. Luis, Technological change and metal demand over time: What can we learn from the past, Sustainable Mater. Technol., 16(2018), p. 54. DOI: 10.1016/j.susmat.2018.02.001
|
|
Z.S. Yu, H.W. Han, P.Y. Feng, S. Zhao, T.Y. Zhou, A. Kakade, S. Kulshrestha, S. Majeed, and X.K. Li, Recent advances in the recovery of metals from waste through biological processes, Bioresour. Technol., 297(2020), art. No. 122416. DOI: 10.1016/j.biortech.2019.122416
|
|
T. Hennebel, N. Boon, S. Maes, and M. Lenz, Biotechnologies for critical raw material recovery from primary and secondary sources: R&D priorities and future perspectives, New Biotechnol., 32(2015), No. 1, p. 121. DOI: 10.1016/j.nbt.2013.08.004
|
|
S.W. Won, P. Kotte, W. Wei, A. Lim, and Y.S. Yun, Biosorbents for recovery of precious metals, Bioresour. Technol., 160(2014), p. 203. DOI: 10.1016/j.biortech.2014.01.121
|
|
J.P.H. Perez, K. Folens, K. Leus, F. Vanhaecke, P. Van Der Voort, and L. Du, Progress in hydrometallurgical technologies to recover critical raw materials and precious metals from low-concentrated streams, Resour. Conserv. Recycl., 142(2019), p. 177. DOI: 10.1016/j.resconrec.2018.11.029
|
|
D.P. Song and J.Q. Xu, Recycling technologies and present legislations of management for waste electrical and electronic equipment, J. Shanghai Sec. Polytech. Univ., 25(2008), No. 2, p. 129.
|
|
W.J. Hall and P.T. Williams, Separation and recovery of materials from scarp printed circuit boards, Resour. Conserv. Recycl., 51(2007), No. 3, p. 691. DOI: 10.1016/j.resconrec.2006.11.010
|
|
Y.J. Ding, S.E. Zhang, B. Liu, H.D. Zheng, C.C. Chang, and C. Ekbergc, Recovery of precious metals from electronic waste and spent catalysts: A review, Resour. Conserv. Recycl., 141(2019), p. 284. DOI: 10.1016/j.resconrec.2018.10.041
|
|
L. Kartal and S. Timur, Direct electrochemical reduction of copper sulfide in molten borax, Int. J. Miner. Metall. Mater., 26(2019), No. 8, p. 992. DOI: 10.1007/s12613-019-1821-x
|
|
J. Mohanty and P.K. Behera, Use of pre-treated TiO2 as cathode material to produce Ti metal through molten salt electrolysis, Trans. Indian Inst. Met., 72(2019), No. 4, p. 859. DOI: 10.1007/s12666-018-1544-0
|
|
S. Masoudifar, M. Bavand-Vandchali, F. Golestani-Fard, and A. Nemati, Molten salt synthesis of a SiC coating on graphite flakes for application in refractory castables, Ceram. Int., 42(2016), No. 10, p. 11951. DOI: 10.1016/j.ceramint.2016.04.120
|
|
J. Zhang, W. Li, Q.L. Jia, L.X. Lin, J.T. Huang, and A.W. Zhang, Molten salt assisted synthesis of 3C–SiC nanowire and its photoluminescence properties, Ceram. Int., 41(2015), No. 10, p. 12614. DOI: 10.1016/j.ceramint.2015.06.089
|
|
Z. Huang, F.L. Li, C.P. Jiao, J.H. Liu, J.T. Huang, L.L. Lu, H.J. Zhang, and S.W. Zhang, Molten salt synthesis of La2Zr2O7 ultrafine powders, Ceram. Int., 42(2016), No. 5, p. 6221. DOI: 10.1016/j.ceramint.2016.01.004
|
|
R.H. Arendt, Liquid-phase sintering of magnetically isotropic and anise by the reaction of BaFe2O4 with Fe2O3, J. Appl. Phys., 44(1973), No. 7, p. 3300. DOI: 10.1063/1.1662750
|
|
Y.M. Li, D. Huang, R.H. Liao, and J.S. Wang, Development of crystal synthesis by molten salt method, J. Ceram., 2(2008), p. 87.
|
|
Q.Q. Yang, Application of molten salt technology, Univ. Chem., 3(1994), p. 1.
|
|
A. Potysz, E.D. van Hullebusch, and J. Kierczak, Perspectives regarding the use of metallurgical slags as secondary metal resources - A review of bioleaching approaches, J. Environ. Manage., 219(2018), p. 138. DOI: 10.1016/j.jenvman.2018.04.083
|
|
K. Pollmann, S. Kutschke, S. Matys, J. Raff, G. Hlawacek, and F.L. Lederer, Bio-recycling of metals: Recycling of technical products using biological applications, Biotechnol. Adv., 36(2018), No. 4, p. 1048. DOI: 10.1016/j.biotechadv.2018.03.006
|
|
X.Y. Yan and D.J. Fray, Molten salt electrolysis for sustainable metals extraction and materials processing: A review, [in] Shing Kuai and Ji Meng, eds., Electrolysis: Theory, Types and Applications, Nova Science, 2010, p. 255.
|
|
T. Guo, S.D. Wang, X.S. Ye, Q. Li, H.N. Liu, M. Guo, and Z.J. Wu, Research progress in the preparation of rare earth alloys by molten salt electrolysis method, Sci. Sin. Chim., 42(2012), No. 9, p. 1328. DOI: 10.1360/032012-252
|
|
J.M. Liu, X.G. Lu, Q. Li, C.T. Chen, H.W. Chen, X.Y. Lü, and G.Z. Zhou, Prospect and retrospect of fused salt electrolysis process for producing refractory metals, Rare Met. Lett., 9(2006), p. 5.
|
|
G.Q. Zong and J.C. Xiao, Advances in the preparation and application of fluoride molten salts, Chem. Ind. Eng. Prog., 37(2018), No. 7, p. 6.
|
|
R. Liu, S.X. Hui, W.J. Ye, Y. Yu, Y.Y. Fu, and X.J. Song, X.G. Deng, Tensile and fracture properties of Ti–62A alloy plate with different microstructures, Rare Met., 31(2012), No. 5, p. 420. DOI: 10.1007/s12598-012-0531-6
|
|
Z. Wang, J. Li, Y.X. Hua, Z. Zhang, Y. Zhang, and P.C. Ke, Research progress in production technology of titanium, Chin. J. Rare Met., 38(2014), No. 5, p. 915.
|
|
M.V. Ginatta, Why produce titanium by EW, JOM., 52(2000), No. 5, p. 18. DOI: 10.1007/s11837-000-0025-0
|
|
M.V. Ginatta, Process for the Electrolytic Production of Metals, US Patent, Appl. US6074545(A), 2000.
|
|
M.V. Ginatta, G. Orsello, and R. Berruti, Method and Cell for the Electrolytic Production of a Polyvalent Metal, US Patent, Appl. US5015342(A), 1991.
|
|
G.Z. Chen, D.J. Fray, and T.W. Farthing, Direct electrochemical reduction of titanium dioxide to titanium in molten calcium chloride, Nature, 407(2000), No. 6802, p. 361. DOI: 10.1038/35030069
|
|
M.F. Liu, S.G Lu, and S.R. Kan, Recent development of electrochemical reduction of oxides to refractory metals and alloys in molten salt, Chin. J. Rare Met., 32(2008), No. 5, p. 130.
|
|
B.J. Zhao, G.H. Cui, and L. Wang, Development of the production of metal and alloy by direct electrochemical removal of oxygen, J. Hebei Polytech. Univ., 30(2008), No. 1, p. 128.
|
|
Z.L. Yu, N. Wang, S. Fang, X.P. Qi, Z.F. Gao, J.Y. Yang, and S.G. Lu, Pilot-plant production of high-performance silicon nanowires by molten salt electrolysis of silica, Ind. Eng. Chem. Res., 59(2020), No. 1, p. 1. DOI: 10.1021/acs.iecr.9b04430
|
|
E. Juzeliūnas and D.J. Fray, Silicon electrochemistry in molten salts, Chem. Rev., 120(2020), No. 3, p. 1690. DOI: 10.1021/acs.chemrev.9b00428
|
|
B.P. Uday, D.E. Woolley, and G.B. Kenney, Emerging SOM technology for the green synthesis of metals from oxides, JOM, 53(2001), No. 10, p. 32. DOI: 10.1007/s11837-001-0053-4
|
|
X.G. Lu, X.L. Zou, C.H. Li, Q.D. Zhong, W.Z. Ding, and Z.F. Zhou, Green electrochemical process solid-oxide oxygen-ionconducting membrane (SOM): Direct extraction of Ti–Fe alloys from natural ilmenite, Metall. Mater. Trans. B, 43(2012), No. 3, p. 503. DOI: 10.1007/s11663-012-9633-7
|
|
X.S. Ye, X.G. Lu, C.H. Li, W.Z. Ding, X.L. Zou, Y.H. Gao, and Q.D. Zhong, Preparation of Ti–Fe based hydrogen storage alloy by SOM method, Int. J. Hydrogen Energy, 36(2011), No. 7, p. 4573. DOI: 10.1016/j.ijhydene.2010.04.098
|
|
F. Cardarelli, Method for Electrowinning of Titanium Metal or Alloy from Titanium Oxide Containing Compound in the Liquid State, US Patent, Appl. US7504017(B2), 2009.
|
|
F. Li, M. Yu, and X.F. Cui, Advances on low cost molten-salt electrolysis technology for Titanium production, Nonferrous Metal., 62(2010), No. 3, p. 96.
|
|
S.Q. Jiao and H.M. Zhu, Novel metallurgical process for titanium production, J. Mater. Res., 21(2006), No. 9, p. 2172. DOI: 10.1557/jmr.2006.0268
|
|
S.Q. Jiao and H.M. Zhu, Electrolysis of Ti2CO solid solution prepared by TiC and TiO2, J. Alloys Compd., 438(2007), No. 1, p. 243.
|
|
R.A. Guidotti and P. Masset, Thermally activated (“thermal”) battery technology Part I: An overview, J. Power Sources, 161(2006), No. 2, p. 1443. DOI: 10.1016/j.jpowsour.2006.06.013
|
|
M.G. Jeong, J.H. Cho, and B.J. Lee, Heat transfer analysis of a high-power and large-capacity thermal battery and investigation of effective thermal model, J. Power Sources, 424(2019), p. 35. DOI: 10.1016/j.jpowsour.2019.03.067
|
|
R.A. Guidotti, F.W. Reinhardt, and J.G. Odinek, Overview of high-temperature batteries for geothermal and oil/gas borehole power sources, J. Power Sources, 136(2004), No. 2, p. 257. DOI: 10.1016/j.jpowsour.2004.03.007
|
|
M.H. Miles, G.E. McManis, and A.N. Fletcher, Effect of temperature and electrolyte composition on the lithium-boron alloy anode in nitrate melts: Passivating films on solid and liquid lithium, Electrochim. Acta, 30(1985), No. 7, p. 889. DOI: 10.1016/0013-4686(85)80146-8
|
|
M.A. Geyer, Thermal storage for solar power plants, Sol. Power Plants, 7(1991), p. 199.
|
|
A.G. Fernández, H. Galleguillos, and F.J. Pérez, Corrosion ability of a novel heat transfer fluid for energy storage in CSP plants, Oxid. Met., 82(2014), No. 5, p. 331.
|
|
T. Bauer, N. Breidenbach, N. Pfleger, D. Laing, and M. Eck, Overview of molten salt storage systems and material development for solar thermal power plants, [in] World Renewable Energy Forum, Colorado, 2(2012), p. 837.
|
|
Y.T. Wu, N. Ren, and Z.F. Ma, Research and application of molten salts for sensible heat storage, Energy Sci. Technol., 2(2013), No. 6, p. 586.
|
|
Z.H. Tian and J. Zhang, Design and research of double-tank molten salt indirect heat storage system for trough solar thermal power generation, Sol. Energy, 22(2012), p. 54.
|
|
A.G. Fernandez, S. Ushak, H. Galleguillos, and F.J. Pérez, Development of new molten salts with LiNO3 and Ca(NO3)2 for energy storage in CSP plants, Appl. Energy, 119(2014), p. 131. DOI: 10.1016/j.apenergy.2013.12.061
|
|
D.L. Zhang, L.M. Liu, M.H. Liu, R.S. Xu, C. Gong, J. Zhang, C.L. Wang, S.Z. Qiu, and G.H. Su, Review of conceptual design and fundamental research of molten salt reactors in China, Int. J. Energy Res., 42(2018), No. 5, p. 1834. DOI: 10.1002/er.3979
|
|
A.C.C. Tseung, Past, present and future of fuel cells, Battery Bimonthly, 32(2002), p. 130.
|
|
S. Frangini and A. Masi, Molten carbonates for advanced and sustainable energy applications: Part Ⅱ. Review of recent literature, Int. J. Hydrogen Energy, 41(2016), No. 42, p. 18971. DOI: 10.1016/j.ijhydene.2016.08.076
|
|
Q.Q. Yang and S.Z. Duan, The new developments of molten salt electrochemistry, Electrochemistry, 7(2001), No. 1, p. 14.
|
|
T. Oishi, K. Koyama, S. Alam, M. Tanaka, and J.C. Lee, Recovery of high purity copper cathode from printed circuit boards using ammoniacal sulfate or chloride solutions, Hydrometallurgy, 89(2007), No. 1, p. 82.
|
|
C.M. Du, C. Shang, X.J. Gong, T. Wang, and X.G. Wei, Plasma methods for metals recovery from metal-containing waste, Waste Manage., 77(2018), p. 373. DOI: 10.1016/j.wasman.2018.04.026
|
|
K. Binnemans, P.T. Jones, B. Blanpain, T. Van Gerven, Y.X. Yang, A. Walton, and M. Buchert, Recycling of rare earths: a critical review, J. Clean. Prod., 51(2013), p. 1. DOI: 10.1016/j.jclepro.2012.12.037
|
|
L.J. Chen, Z.L. Li, A. Gong, L. Tian, and Z.F. Xu, Research progress of rare earth recovery from rare earth waste, J. Chin. Soc. Rare Earths, 37(2019), No. 3, p. 259.
|
|
A. Abbasalizadeh, L. Teng, S. Seetharaman, J. Sietsma, and Y. Yang, Rare earth extraction from NdFeB magnets and rare earth oxides using aluminum chloride/fluoride molten salts, [in] Ismar Borges De Lima and Walter Leal Filho, eds., Rare Earths Industry, Elsevier Inc, Netherlands, 2016, p. 357.
|
|
Z.S. Hua, J. Wang, L. Wang, Z. Zhao, X.L. Li, Y.P. Xiao, and Y.X. Yang, Selective extraction of rare earth elements from NdFeB scrap by molten chlorides, ACS Sustainable Chem. Eng., 2(2014), No. 11, p. 2536. DOI: 10.1021/sc5004456
|
|
A. Abbasalizadeh, A. Malfliet, S. Seetharaman, J. Sietsma, and Y.X. Yang, Electrochemical recovery of rare earth elements from magnets: conversion of rare earth based metals into rare earth fluorides in molten salts, Mater. Trans., 58(2017), No. 3, p. 400. DOI: 10.2320/matertrans.MK201617
|
|
A. Abbasalizadeh, A. Malfliet, S. Seetharaman, J. Sietsma, and Y.X. Yang, Electrochemical extraction of rare earth metals in molten fluorides: conversion of rare earth oxides into rare earth fluorides using fluoride additives, J. Sustainable Metall., 3(2017), No. 3, p. 627. DOI: 10.1007/s40831-017-0120-x
|
|
M. Tanaka, T. Oki, K. Koyama, H. Narita, and T. Oishi, Recycling of rare Earths from Scrap, [in] Handbook on the Physics and Chemistry of Rare Earths, Elsevier, Amsterdam, 2015.
|
|
A.M. Martinez, O. Kjos, E. Skybakmoen, A. Solheim, and G.M. Haarberg, Extraction of rare earth metals from Nd-based scrap by electrolysis from molten salts, ECS Trans., 50(2012), No. 11, p. 453.
|
|
K. Yasuda, S. Kobayashi, T. Nohira, and R. Hagiwara, Electrochemical formation of Dy–Ni alloys in molten NaCl–KCl–DyCl3, Electrochim. Acta, 106(2013), p. 293. DOI: 10.1016/j.electacta.2013.05.095
|
|
K. Yasuda, S. Kobayashi, T. Nohira, and R. Hagiwara, Electrochemical formation of Nd–Ni alloys in molten NaCl–KCl–NdCl3, Electrochim. Acta, 92(2013), p. 349. DOI: 10.1016/j.electacta.2013.01.049
|
|
H. Konishi, H. Ono, T. Oishi, and T. Nohira, Separation of Dy from model magnet scraps using molten salt electrochemical process, J. Jpn. Soc. Exp. Mech., 12(2012), p. s243. DOI: 10.11395/jjsem.12.s243
|
|
H. Konishi, H. Ono, E. Takeuchi, T. Nohira, and T. Oishi, Separation of Dy from Nd–Fe–B magnet scraps using molten salt electrolysis, ECS Trans., 64(2014), No. 4, p. 593. DOI: 10.1149/06404.0593ecst
|
|
Y. Kamimoto, T. Itoh, K. Kuroda, and R. Ichino, Recovery of rare-earth elements from neodymium magnets using molten salt electrolysis, J. Mater. Cycles Waste Manage., 19(2017), No. 3, p. 1017. DOI: 10.1007/s10163-016-0563-3
|
|
Y. Kamimoto, G. Yoshimura, T. Itoh, K. Kuroda, and R. Ichino, Leaching of rare earth elements from neodymium magnet using electrochemical method, Trans. Mater. Res. Soc. Jpn., 40(2015), No. 4, p. 343. DOI: 10.14723/tmrsj.40.343
|
|
Y.S. Yang, C.Q. Lan, L.Y. Guo, Z.Q. An, Z.W. Zhao, and B.W. Li, Recovery of rare-earth element from rare-earth permanent magnet waste by electro-refining in molten fluorides, Sep. Purif. Technol., 233(2020), art. No. 116030. DOI: 10.1016/j.seppur.2019.116030
|
|
D. Vaden, S.X. Li, B.R. Westphal, K.B. Davies, T.A. Johnson, and D.M. Pace, Engineering-scale liquid cadmium cathode experiments, Nucl. Technol., 162(2008), No. 2, p. 124. DOI: 10.13182/NT08-A3938
|
|
E.Y. Choi and S.M. Jeong, Electrochemical processing of spent nuclear fuels: An overview of oxide reduction in pyroprocessing technology, Prog. Nat. Sci. Mater., 25(2015), No. 6, p. 572. DOI: 10.1016/j.pnsc.2015.11.001
|
|
H. Tang, Y.M. Ren, L. Shao, Y. Zhong, and R. Gao, Development of pyroprocessing of spent nuclear fuel by molten salts electrolysis, J. Nucl. Radiochem., 39(2017), No. 6, p. 385.
|
|
J.M. Hur, C.S. Seo, S.S. Hong, D.S. Kang, and S.W. Park, Metallization of U3O8 via catalytic electrochemical reduction with Li2O in LiCl molten salt, React. Kinet. Catal. Lett., 80(2003), No. 2, p. 217. DOI: 10.1023/B:REAC.0000006128.15961.1d
|
|
S.B. Park, B.H. Park, S.M. Jeong, J.M. Hur, C.S. Seo, S.H. Choi, and S.W. Park, Characteristics of an integrated cathode assembly for the electrolytic reduction of uranium oxide in a LiCl–Li2O molten salt, J. Radioanal. Nucl. Chem., 268(2006), No. 3, p. 489. DOI: 10.1007/s10967-006-0196-4
|
|
S.M. Jeong, S.B. Park, S.S. Hong, C.S. Seo, and S.W. Park, Electrolytic production of metallic uranium from U3O8 in a 20-kg batchscale reactor, J. Radioanal. Nucl. Chem., 268(2006), No. 2, p. 349. DOI: 10.1007/s10967-006-0172-z
|
|
Y. Sakamura and T. Omori, Electrolytic reduction and electrorefining of uranium to develop pyrochemical reprocessing of oxide fuels, Nucl. Technol., 171(2010), No. 3, p. 266. DOI: 10.13182/NT10-A10861
|
|
E.Y. Choi, J.W. Lee, J.J. Park, J.M. Hur, J.K. Kim, K.Y. Jung, and S.M. Jeong, Electrochemical reduction behavior of a highly porous SIMFUEL particle in a LiCl molten salt, Chem. Eng. J., 207-208(2012), p. 514.
|
|
E.Y. Choi, J.M. Hur, I.K. Choi, S.G. Kwon, D.S. Kang, S.S. Hong, H.S. Shin, M.A. Yoo, and S.M. Jeong, Electrochemical reduction of porous 17 kg uranium oxide pellets by selection of an optimal cathode/anode surface area ratio, J. Nucl. Mater., 418(2011), No. 1-3, p. 87.
|
|
Y. Shin, I. Kim, S. Oh, C. Park, and C. Lee, Lithium recovery from radioactive molten salt wastes by electrolysis, J. Radioanal. Nucl. Chem., 243(2000), No. 3, p. 639. DOI: 10.1023/A:1010601816105
|
|
J.S. Zhang, Electrochemistry of actinides and fission products in molten salts-Data review, J. Nucl. Mater., 447(2014), No. 1-3, p. 271. DOI: 10.1016/j.jnucmat.2013.12.017
|
|
K. Kinoshita, T. Inoue, S.P. Fusselman, D.L. Grimmett, C.L. Krueger, and T.S. Storvick, Electrodeposition of uranium and transuranic elements onto solid cathode in LiCl–KCl/Cd system for pyrometallurgical partitioning, J. Nucl. Sci. Technol., 40(2003), No. 7, p. 524. DOI: 10.1080/18811248.2003.9715387
|
|
S.W. Kwon, D.H. Ahn, E.H. Kim, and H.G. Ahn, A study on the recovery of actinide elements from molten LiCl–KCl eutectic salt by an electrochemical separation, J. Ind. Eng. Chem., 15(2009), No. 1, p. 86. DOI: 10.1016/j.jiec.2008.08.006
|
|
J. Park, S. Choi, S. Sohn, K.R. Kim, and I.S. Hwanga, Cyclic voltammetry on zirconium redox reactions in LiCl–KCl–ZrCl4 at 500°C for electrorefining contaminated zircaloy-4 cladding, J. Electrochem. Soc., 161(2014), No. 3, p. H97. DOI: 10.1149/2.046403jes
|
|
C.H. Lee, K.H. Kang, M.K. Jeon, C.M. Heo, and Y.L. Lee, Electroreflning of zirconium from zircaloy-4 cladding hulls in LiCl–KCl molten salts, J. Electrochem. Soc., 159(2012), No. 8, p. D463. DOI: 10.1149/2.012208jes
|
|
Y.L. Lee, C.H. Lee, M.K. Jeon, and K.H. Kang, Studies on the electrochemical dissolution for the treatment of 10 g-scale zircaloy-4 cladding hull wastes in LiCl–KCl molten salts, J. Korean Radioact. Waste Soc., 10(2012), No. 4, p. 273. DOI: 10.7733/jkrws.2012.10.4.273
|
|
C.H. Lee, M.K. Jeon, C.M. Heo, Y.L. Lee, K.H. Kang, and G.I. Park, Effect of Zr oxide on the electrochemical dissolution of zircaloy-4 cladding tubes, J. Electrochem. Soc., 159(2012), No. 11, p. E171. DOI: 10.1149/2.031212jes
|
|
C.H. Lee, Y.L. Lee, M.K. Jeon, Y.T. Choi, K.H. Kang, and G.I. Park, Effects of pretreatment processes for Zr electrorefining of oxidized Zircaloy-4 cladding tubes, J. Nucl. Mater., 449(2014), No. 1-3, p. 93. DOI: 10.1016/j.jnucmat.2014.02.034
|
|
R. Fujita, H. Nakamura, K. Mizuguchi, M. Sato, T. Shibano, Y. Ito, T. Goto, T. Terai, and S. Ogawa, Zirconium recovery process for spent zircaloy components from light water reactor (LWR) by electrorefining in molten salts, Electrochemistry, 73(2005), No. 8, p. 751. DOI: 10.5796/electrochemistry.73.751
|
|
T. Goto, T. Nohira, R. Hagiwara, and Y. Ito, Selected topics of molten fluorides in the field of nuclear engineering, J. Fluorine Chem., 130(2009), No. 1, p. 102. DOI: 10.1016/j.jfluchem.2008.07.016
|
|
C.H. Lee, D.Y. Kang, M.K. Jeon, K.H. Kang, S.W. Paek, D.H. Ahn, and K.T. Park, Addition effect of fluoride compounds for Zr electrorefining in LiCl–KCl molten salts, Int. J. Electrochem. Sci., 11(2016), p. 566.
|
|
Y. Akai and R. Fujita, Development of transuranium element recovery from high-level radioactive liquid waste, J. Nucl. Sci. Technol., 33(1996), No. 10, p. 807. DOI: 10.1080/18811248.1996.9732007
|
|
L. Cassayre, P. Palau, P. Chamelot, and L. Massot, Properties of low-temperature melting electrolytes for the aluminum electrolysis process: A review, J. Chem. Eng. Data, 55(2010), No. 11, p. 4549. DOI: 10.1021/je100214x
|
|
M. Ueda, S. Tsukamoto, S. Konda, and T. Ohtsuka, Recovery of aluminum from oxide particles in aluminum dross using AlF3-NaF-BaCl2 molten salt, J. Appl. Electrochem., 35(2005), No. 9, p. 925. DOI: 10.1007/s10800-005-5289-1
|
|
M. Ueda, M. Amemiya, T. Ishikawa, and T. Ohtsuka, Recovery of aluminum alloy from aluminum dross by treatment of chloride-fluoride mixture melt, J. Jpn. Inst. Met., 63(1999), No. 3, p. 279. DOI: 10.2320/jinstmet1952.63.3_279
|
|
X.Y. Yan, Chemical and electrochemical processing of aluminum dross using molten salts, Metall. Mater. Trans. B, 39(2008), No. 2, p. 348. DOI: 10.1007/s11663-008-9135-9
|
|
L. Xu, Y. Xiao, A. van Sandwijk, Q. Xu, and Y. Yang, Production of nuclear grade zirconium: A review, J. Nucl. Mater., 466(2015), p. 21. DOI: 10.1016/j.jnucmat.2015.07.010
|
|
K.T. Park, T.H. Lee, N.C. Jo, H.H. Nersisyan, B.S. Chun, H.H. Lee, and J.H. Lee, Purification of nuclear grade Zr scrap as the high purity dense Zr deposits from Zirlo scrap by electrorefining in LiF–KF–ZrF4 molten fluorides, J. Nucl. Mater., 436(2013), No. 1-3, p. 130. DOI: 10.1016/j.jnucmat.2013.01.310
|
|
D.J. Park, S.H. Kim, K.T. Park, J.H. Mun, H.H. Lee, and J.H. Lee, Electrorefining behavior of zirconium scrap with multiple cathode in fluoride-based molten salt, J. Nucl. Fuel Cycle Waste Technol., 13(2015), No. 1, p. 11. DOI: 10.7733/jnfcwt.2015.13.1.11
|
|
X.L. Zou and X.G. Lu, Preparation of titanium alloy by direct reduction of Ti-bearing blast furnace slag, Chin. J. Nonferrous Met., 20(2010), No. 9, p. 1829.
|
|
X.L. Zou, X.G. Lu, W. Xiao, Z.F. Zhou, Q.D. Zhong, and W.Z. Ding, Direct electrochemical extraction of Ti5Si3 from Ti/Si-containing metal oxide compounds in molten CaCl2, J. Shanghai Jiaotong Univ., 18(2013), p. 111. DOI: 10.1007/s12204-013-1373-6
|
|
X.L. Zou, X.G. Lu, Z.F. Zhou, C.H. Li, and W.Z. Ding, Direct selective extraction of titanium silicide Ti5Si3 from multi-component Ti-bearing compounds in molten salt by an electrochemical process, Electrochim. Acta, 56(2011), No. 24, p. 8430. DOI: 10.1016/j.electacta.2011.07.026
|
|
S.H. Li, X.L. Zou, K. Zheng, X.G. Lu, C.Y. Chen, X. Li, Q. Xu, and Z.F. Zhou, Electrosynthesis of Ti5Si3, Ti5Si3/TiC, and Ti5Si3/Ti3SiC2 from Ti-bearing blast furnace slag in molten CaCl2, Metall. Mater. Trans. B, 49(2018), No. 2, p. 790. DOI: 10.1007/s11663-018-1192-0
|
|
D. Mishra, S. Sinha, K. Sahu, A. Agrawal, and R. Kumar, Recycling of secondary tungsten resources, Trans. Indian Inst. Met., 70(2017), No. 2, p. 479. DOI: 10.1007/s12666-016-1003-8
|
|
P.K. Meherotra, Reduction of environmental impact in hardmetal technologies, Met. Powder Rep., 72(2017), No. 4, p. 267. DOI: 10.1016/j.mprp.2016.02.052
|
|
R. Srivastava, J. Lee, M. Bae, and V. Kumar, Reclamation of tungsten from carbide scraps and spent materials, J. Mater. Sci., 54(2019), No. 1, p. 83. DOI: 10.1007/s10853-018-2876-1
|
|
A. Shemi, A. Magumise, S. Ndlovu, and N. Sacks, Recycling of tungsten carbide scrap metal: A review of recycling methods and future prospects, Miner. Eng., 122(2018), p. 195. DOI: 10.1016/j.mineng.2018.03.036
|
|
T. Kojima, T. Shimizu, R. Sasai, and H. Itoh, Recycling process of WC–Co cermets by hydrothermal treatment, J. Mater. Sci., 40(2005), No. 19, p. 5167. DOI: 10.1007/s10853-005-4407-0
|
|
C. Edtmaier, R. Schiesser, C. Meissl, W.D. Schubert, A. Bock, A. Schoen, and B. Zeiler, Selective removal of the cobalt binder in WC/Co based hardmetal scraps by acetic acid leaching, Hydrometallurgy, 76(2005), No. 1-2, p. 63. DOI: 10.1016/j.hydromet.2004.09.002
|
|
C.S. Freemantle, N. Sacks, M. Topic, and C.A. Pineda-Vargas, PIXE characterization of byproducts resulting from the zinc recycling of industrial cemented carbides, Nucl. Instrum. Methods Phys. Res., 363(2015), p. 167. DOI: 10.1016/j.nimb.2015.07.064
|
|
B.X. Liu, A.H. Shi, Q. Su, G.J. Chen, W. Li, L.N. Zhang, and B. Yang, Recovery of tungsten carbides to prepare the ultrafine WC–Co composite powder by two-step reduction process, Powder Technol., 306(2016), p. 113.
|
|
X.L. Xi, G.H. Si, Z.R. Nie, and L.W. Ma, Electrochemical behavior of tungsten ions from WC scrap dissolution in a chloride melt, Electrochim. Acta, 184(2015), p. 233. DOI: 10.1016/j.electacta.2015.10.071
|
|
G.H. Si, X.L. Xi, Z.R. Nie, L.W. Zhang, and L.W. Ma, Preparation and characterization of tungsten nanopowders from WC scrap in molten salts, Int. J. Refract. Met. H., 54(2016), p. 422. DOI: 10.1016/j.ijrmhm.2015.10.002
|
|
Q.H. Zhang, X.L. Xi, Z.R. Nie, L.W. Zhang, and L.W. Ma, Electrochemical dissolution of cemented carbide scrap and electrochemical preparation of tungsten and cobalt metals, Int. J. Refract. Met. Hard Mater., 79(2019), p. 145. DOI: 10.1016/j.ijrmhm.2018.12.001
|
|
X.J. Xiao, X.L. Xi, Z.R. Nie, L.W. Zhang, and L.W. Ma, Direct electrochemical preparation of cobalt, tungsten, and tungsten carbide from cemented carbide scrap, Metall. Mater. Trans. B, 48(2017), No. 1, p. 692. DOI: 10.1007/s11663-016-0836-1
|
|
L.W. Zhang, Z.R. Nie, X.L. Xi, L.W. Ma, X.J. Xiao, and M. Li, Electrochemical dissolution of tungsten carbide in NaCl-KCl-Na2WO4 molten salt, Metall. Mater. Trans. B, 49(2018), No. 1, p. 334. DOI: 10.1007/s11663-017-1125-3
|
|
L.W. Zhang, Z.R. Nie, X.L. Xi, and L.W. Ma, Electrochemical separation and extraction of cobalt and tungsten from cemented scrap, Sep. Purif. Technol., 195(2018), p. 244. DOI: 10.1016/j.seppur.2017.12.022
|
|
M. Li, X.L. Xi, Z.R. Nie, L.W. Ma, and Q.Q. Liu, Electrochemical extraction of tungsten derived from WC scrap and electrochemical properties of tungsten ion in LiCl–KCl molten salt, J. Electrochem. Soc., 163(2016), No. 13, p. D728. DOI: 10.1149/2.1131613jes
|
|
M. Li, Electrochemical studies on the reduction behavior of Co2+ in eutectic NaF–KF melt, Int. J. Electrochem. Sci., 13(2018), p. 4208.
|
|
X.L. Xi, Q.Q. Liu, Z.R. Nie, M. Li, and L.W. Ma, Electrochemical preparation of tungsten and cobalt from cemented carbide scrap in NaF-KF molten salts, Int. J. Refract. Met. Hard Mater., 70(2018), p. 77. DOI: 10.1016/j.ijrmhm.2017.09.009
|
|
M. Li, X.L. Xi, Z.R. Nie, L.W. Ma, and Q.Q. Liu, Recovery of tungsten from WC–Co hard metal scraps using molten salts electrolysis, J. Mater. Res. Technol., 8(2019), No. 1, p. 1440. DOI: 10.1016/j.jmrt.2018.10.010
|
|
L.W. Zhang, Z.R. Nie, and X.L. Xi, Preparation of tungsten nanoparticles from spent tungsten carbide by molten salt electrolysis, Mater. Sci. Forum, 913(2018), p. 961. DOI: 10.4028/www.scientific.net/MSF.913.961
|
| [1] | Aliakbar Ghadi, Hassan Saghafian, Mansour Soltanieh, Zhi-gang Yang. Diffusion mechanism in molten salt baths during the production of carbide coatings via thermal reactive diffusion [J]. International Journal of Minerals, Metallurgy and Materials, 2017, 24(12): 1448-1458. DOI: 10.1007/s12613-017-1538-7 |
| [2] | Chun-fa Liao, Yun-fen Jiao, Xu Wang, Bo-qing Cai, Qiang-chao Sun, Hao Tang. Electrical conductivity optimization of the Na3AlF6-Al2O3-Sm2O3 molten salts system for Al-Sm intermediate binary alloy production [J]. International Journal of Minerals, Metallurgy and Materials, 2017, 24(9): 1034-1042. DOI: 10.1007/s12613-017-1493-3 |
| [3] | Tao Yang, Peng-long Qiu, Mei Zhang, Kuo-Chih Chou, Xin-mei Hou, Bai-jun Yan. Molten salt synthesis of mullite nanowhiskers using different silica sources [J]. International Journal of Minerals, Metallurgy and Materials, 2015, 22(8): 884-891. DOI: 10.1007/s12613-015-1146-3 |
| [4] | Shi-lu Zhao, Jun Zhang, Zhen Zhang, Shuang-hong Wang, Zheng-gui Zhang. Microstructure and mechanical properties of (Ti,Al,Zr)N/(Ti,Al,Zr,Cr)N films on cemented carbide substrates [J]. International Journal of Minerals, Metallurgy and Materials, 2014, 21(1): 77-81. DOI: 10.1007/s12613-014-0868-y |
| [5] | Liu-yong Shi, Yi-min Liu, Ji-hua Huang, Shou-quan Zhang, Xing-ke Zhao. Growth kinetics of cubic carbide free layers in graded cemented carbides [J]. International Journal of Minerals, Metallurgy and Materials, 2012, 19(1): 64-71. DOI: 10.1007/s12613-012-0516-3 |
| [6] | Tian-en Yang, Ji Xiong, Lan Sun, Zhi-xing Guo, Ding Cao. Effect of nitrogen introduction methods on the microstructure and properties of gradient cemented carbides [J]. International Journal of Minerals, Metallurgy and Materials, 2011, 18(6): 709-716. DOI: 10.1007/s12613-011-0501-2 |
| [7] | Li Chen, Enxi Wu, Fei Yin, Jia Li. Effects of gradient structure on the microstructures and properties of coated cemented carbides [J]. International Journal of Minerals, Metallurgy and Materials, 2006, 13(4): 363-367. DOI: 10.1016/S1005-8850(06)60075-3 |
| [8] | Xiaoliang Shi, Gangqin Shao, Xinglong Duan, Runzhang Yuan. Atomic force microscope study of WC-10Co cemented carbide sintered from nanocrystalline composite powders [J]. International Journal of Minerals, Metallurgy and Materials, 2005, 12(6): 558-563. |
| [9] | Ying Sing Fung, Ruqi Zhou. Physical and Electrochemical Properties of Acid 1-Methyl-3-Ethylimidazolium Chloride/AlCl3/LiAlCl4Molten Salt [J]. International Journal of Minerals, Metallurgy and Materials, 2000, 7(4): 286-291. |
| [10] | Binghai Liu, Yue Zhang, Shixi Ouyang, Qikai Li. Manufacture of the Ultrafine Grain WC/Co Cemented Carbides by Combined Sintering Processing [J]. International Journal of Minerals, Metallurgy and Materials, 1999, 6(3): 208-213. |
| 1. | Youpeng Xu, Sheng Pang, Liangwei Cong, et al. Overview of in-situ oxygen production technologies for lunar resources. International Journal of Minerals, Metallurgy and Materials, 2025, 32(2): 233. DOI:10.1007/s12613-024-2925-5 |
| 2. | Rongcen Zhao, Yuqing Xu, Zepeng Lv, et al. Structure Design of Electrolytic Cell: An Approach of Enhancing Mass Transfer. Journal of The Electrochemical Society, 2025, 172(3): 034512. DOI:10.1149/1945-7111/adbfc7 |
| 3. | A. Birri, R. Chesser, N. Termini, et al. Reference Correlations for the Density and Viscosity of Molten Alkali and Alkaline Earth Fluoride Salts. Journal of Physical and Chemical Reference Data, 2025, 54(2) DOI:10.1063/5.0264576 |
| 4. | Shaolong Li, Zepeng Lv, Jilin He, et al. Anodic dissolution of consumable ZrC O and cathodic deposition of zirconium in NaCl-KCl based melts. Journal of Molecular Liquids, 2025, 420: 126831. DOI:10.1016/j.molliq.2024.126831 |
| 5. | Ali Zakeri, Leili Tafaghodi. A Review of the Current Progress in High-Temperature Recycling Strategies for Recovery of Rare-Earth Elements from Magnet Waste. Journal of Sustainable Metallurgy, 2025, 11(1): 88. DOI:10.1007/s40831-025-01010-9 |
| 6. | Chongrui Zhuang, Tianqin Liu, Terigele Terigele, et al. Preparation of Uniform Molybdenum Films on Nickel Plate and Nickel Foam Using Electrochemical Deposition in Molten Salt. Journal of The Electrochemical Society, 2025, 172(5): 052502. DOI:10.1149/1945-7111/add214 |
| 7. | Hongzhan Lv, Liwen Zhang, Xiaoli Xi, et al. Electrochemical dissolution, reduction, and nucleation mechanisms of molybdenum in NaCl-KCl molten salt systems. Journal of Materials Science & Technology, 2025, 238: 45. DOI:10.1016/j.jmst.2025.01.085 |
| 8. | Hongzhan Lv, Liwen Zhang, Xiaoli Xi, et al. Study on separation and purification of titanium alloys (TC4-6Al-4V) by molten salt electrolysis. Separation and Purification Technology, 2025, 354: 129213. DOI:10.1016/j.seppur.2024.129213 |
| 9. | Michael Girma Haile, Alabi Oladunni Oyelola, Fatai Olufemi Aramide, et al. Hydrometallurgical, pyrometallurgical and electrometallurgical extraction of niobium and tantalum: an overview. Mineral Processing and Extractive Metallurgy: Transactions of the Institutions of Mining and Metallurgy, 2025, 134(1): 3. DOI:10.1177/25726641241301982 |
| 10. | Huiling Jiao, Yangsi Liu, Xiaoli Xi, et al. Synthesis of Bi2WxMo1−xO6/BiOBr composites with superior photocatalytic activity by one-pot method from secondary resources. Applied Surface Science, 2025, 695: 162937. DOI:10.1016/j.apsusc.2025.162937 |
| 11. | Xinda Wang, Liwen Zhang, Liwen Ma, et al. First principles modeling of WO3 transport in molten Na2WO4 via a grotthuss-like mechanism and the kinetic characterization involved. Journal of Molecular Liquids, 2024, 412: 125747. DOI:10.1016/j.molliq.2024.125747 |
| 12. | Xuena Men, Zepeng Lv, Shaolong Li, et al. Controlled synthesis of tantalum-based solid solution and exploration of electrochemical properties as soluble anode for molten salt electrolysis. International Journal of Refractory Metals and Hard Materials, 2024, 125: 106888. DOI:10.1016/j.ijrmhm.2024.106888 |
| 13. | Tianzhu Mu, Fuxing Zhu, Yan Zhao, et al. Preparation of Titanium Metal by Deoxygenation Under KCl-NaCl-YCl3 System Using Soluble Anode. Metals, 2024, 14(11): 1288. DOI:10.3390/met14111288 |
| 14. | Jiale Zhang, Liwen Zhang, Xiaoli Xi, et al. Electrochemical separation technology and mechanism of tungsten and cobalt in Na2WO4-WO3-CoO molten salts. Separation and Purification Technology, 2024, 330: 125270. DOI:10.1016/j.seppur.2023.125270 |
| 15. | Xuena Men, Shaolong Li, Zepeng Lv, et al. Kinetic analysis of the cathodic reduction processes in molten salt electrolysis. Journal of Alloys and Compounds, 2024, 1004: 175785. DOI:10.1016/j.jallcom.2024.175785 |
| 16. | Xinda Wang, Liwen Zhang, Liwen Ma, et al. Multiscale modeling of Na2WO4–WO3–CoO melt diffusion layer for molecular dynamics combined with finite element analysis. Materials Chemistry and Physics, 2024, 311: 128589. DOI:10.1016/j.matchemphys.2023.128589 |
| 17. | Timothy Lichtenstein, Jarrod Gesualdi, Sarah A. Stariha, et al. Electrolytic recovery of metals from lithium battery cathodes in moisture-tolerant molten hydroxide salt. Electrochimica Acta, 2024, 503: 144866. DOI:10.1016/j.electacta.2024.144866 |
| 18. | Umanga De Silva, Timothy P. Coons. Molten Salt Electrodeposition: Review. Energies, 2024, 17(15): 3832. DOI:10.3390/en17153832 |
| 19. | Lin-Lin Wang, Han-Dong Jiao, Rui Yuan, et al. Electrodeposition of rare refractory metals in low-temperature ionic liquids. Rare Metals, 2024, 43(10): 4921. DOI:10.1007/s12598-024-02787-6 |
| 20. | Takuya Tamura, Mingjun Li. Upgrading recycling of Al–7 wt%Si alloys using electromagnetic force in directional solidification. Journal of Alloys and Compounds, 2024, 979: 173528. DOI:10.1016/j.jallcom.2024.173528 |
| 21. | Vahe Gharakhanyan, Luke J. Wirth, Jose A. Garrido Torres, et al. Discovering melting temperature prediction models of inorganic solids by combining supervised and unsupervised learning. The Journal of Chemical Physics, 2024, 160(20) DOI:10.1063/5.0207033 |
| 22. | Shu-Xuan Yan, You-Zhou Jiang, Xiang-Ping Chen, et al. Engineering classification recycling of spent lithium-ion batteries through pretreatment: a comprehensive review from laboratory to scale-up application. Rare Metals, 2024, 43(3): 915. DOI:10.1007/s12598-023-02377-y |
| 23. | Yun Xie, Min Bu, Guimin Lu. Insights into CaCl2-NaCl-KCl molten salt: A machine learning approach to unraveling structure and properties. Journal of Energy Storage, 2024, 102: 114156. DOI:10.1016/j.est.2024.114156 |
| 24. | Junhan Luo, Jingcui Liu, Qi Qing, et al. Real-Time Monitoring of Metal Ions during the Molten Salt Electrolysis Process by Optical Emission Spectrometry Based on Microplasma. Analytical Chemistry, 2024, 96(52): 20406. DOI:10.1021/acs.analchem.4c04004 |
| 25. | A. M. Amirov, M. A. Akhmedov, Z. Yu. Kubataev, et al. Effect of lithium perchlorate addition on LiNO3–KNO3 nitrate eutectic. Ionics, 2024, 30(10): 6089. DOI:10.1007/s11581-024-05715-x |
| 26. | Lun-Ao Ouyang, Yapeng He, Puqiang He, et al. Emerging Electrochemical Techniques for Recycling Spent Lead Paste in Lead-Acid Batteries. Journal of Sustainable Metallurgy, 2024, 10(4): 1905. DOI:10.1007/s40831-024-00928-w |
| 27. | Zaoming Chen, Ruzhen Peng, Zhen Xiang, et al. Low-Acid Leaching for Preferential Lithium Extraction and Preparation of Lithium Carbonate from Rare Earth Molten Salt Electrolytic Slag. Metals, 2024, 14(11): 1303. DOI:10.3390/met14111303 |
| 28. | Junhan Luo, Qi Qing, Zhe Wang, et al. Electrodeposition of UO2 nanoparticles in molten salt with microplasma gaseous cathode. Separation and Purification Technology, 2024, 332: 125829. DOI:10.1016/j.seppur.2023.125829 |
| 29. | Xiaoyu Shi, Chongxiao Guo, Jiamiao Ni, et al. Growth kinetics of titanium carbide coating by molten salt synthesis process on graphite sheet surface. International Journal of Minerals, Metallurgy and Materials, 2024, 31(8): 1858. DOI:10.1007/s12613-023-2749-8 |
| 30. | Zhaoqing Cai, Huixiu Chen, Meng Gao, et al. Quantification of lithium in molten chlorides by optical emission spectrometry using a novel molten-salt-electrode microplasma source. Talanta, 2024, 266: 125111. DOI:10.1016/j.talanta.2023.125111 |
| 31. | Hui Li, Xuexue Wei, Jinglong Liang, et al. Molecular dynamics simulations of the local structure and physicochemical properties of CaCl2molten salt. International Journal of Chemical Reactor Engineering, 2024, 22(4): 447. DOI:10.1515/ijcre-2023-0228 |
| 32. | Pengwei Li, Shaohua Luo, Yicheng Lin, et al. Fundamentals of the recycling of spent lithium-ion batteries. Chemical Society Reviews, 2024, 53(24): 11967. DOI:10.1039/D4CS00362D |
| 33. | Zhongyue Zhang, Yuan Ji, Qiu Jiang, et al. Molten-salt synthesized MXene for catalytic applications: A review. Chemical Physics Reviews, 2024, 5(3) DOI:10.1063/5.0215613 |
| 34. | Sanghyeok Im, Jarrod Gesualdi, Minkyu Kim, et al. Thermodynamic properties of Nd-Fe alloys via emf measurements in LiCl-KCl-NdCl3 electrolyte. Journal of Alloys and Compounds, 2024, 970: 172650. DOI:10.1016/j.jallcom.2023.172650 |
| 35. | Buju Guo, Yaowu Wang, Yuyao Huang, et al. Upcycling of scrap aluminum to pure aluminum through molten salt electrolysis. Process Safety and Environmental Protection, 2024, 191: 94. DOI:10.1016/j.psep.2024.07.046 |
| 36. | Timothy Lichtenstein, Colin V. Patricelli, Krista L. Hawthorne. Application of the Solute-Solvent EMF Cell to Measure Activity of NiF2 in Molten FLiNaK. Journal of The Electrochemical Society, 2023, 170(6): 066506. DOI:10.1149/1945-7111/acd9f2 |
| 37. | Zhongya Pang, Xueqiang Zhang, Conghui Hu, et al. Electrosynthesis and Evaluation of Homogeneous CoCrNi Medium-Entropy Alloys for Structural Applications. Metallurgical and Materials Transactions B, 2023, 54(5): 2824. DOI:10.1007/s11663-023-02877-3 |
| 38. | Xin Song, Shaolong Li, Shanshan Liu, et al. Coordination states of metal ions in molten salts and their characterization methods. International Journal of Minerals, Metallurgy and Materials, 2023, 30(7): 1261. DOI:10.1007/s12613-023-2608-7 |
| 39. | Li Ding, Shanxin Yang, Yongde Yan, et al. Electrochemical and kinetic analysis of Ce recovery using Ga electrode in LiCl-KCl melt. Separation and Purification Technology, 2023, 323: 124492. DOI:10.1016/j.seppur.2023.124492 |
| 40. | Ming Li, Chuanying Liu, Anting Ding, et al. A review on the extraction and recovery of critical metals using molten salt electrolysis. Journal of Environmental Chemical Engineering, 2023, 11(3): 109746. DOI:10.1016/j.jece.2023.109746 |
| 41. | Hao Wu, Huashan Yan, Yanzhen Liang, et al. Rare earth recovery from fluoride molten-salt electrolytic slag by sodium carbonate roasting-hydrochloric acid leaching. Journal of Rare Earths, 2023, 41(8): 1242. DOI:10.1016/j.jre.2022.07.001 |
| 42. | Can Li, N. Clament Sagaya Selvam, Jiye Fang. Shape-Controlled Synthesis of Platinum-Based Nanocrystals and Their Electrocatalytic Applications in Fuel Cells. Nano-Micro Letters, 2023, 15(1) DOI:10.1007/s40820-023-01060-2 |
| 43. | Haoyu Zhang, Baoguo Zhang, Bing Ai, et al. Direct electrochemical extraction of metallic Li from a molecular liquid–Based electrolyte under ambient conditions. Electrochimica Acta, 2023, 441: 141845. DOI:10.1016/j.electacta.2023.141845 |
| 44. | Malik M. Gafurov, Kamil Sh. Rabadanov. High-temperature vibrational spectroscopy of molten electrolytes. Applied Spectroscopy Reviews, 2023, 58(7): 489. DOI:10.1080/05704928.2022.2048305 |
| 45. | Hang Lu, Liwen Zhang, Xiaoli Xi, et al. Optimization of pulse bi-directional electrolysis in-situ synthesis of tungsten carbide by response surface methodology. International Journal of Refractory Metals and Hard Materials, 2023, 111: 106063. DOI:10.1016/j.ijrmhm.2022.106063 |
| 46. | Hui Li, Lingyue Song, Jinglong Liang, et al. Study on the Influence of CaO on the Electrochemical Reduction of Fe2O3 in NaCl-CaCl2 Molten Salt. Molecules, 2023, 28(24): 8103. DOI:10.3390/molecules28248103 |
| 47. | Namhyeok Kim, Seohae Kim, Seongwoo Jeong, et al. Seawater to resource technologies with NASICON solid electrolyte: a review. Frontiers in Batteries and Electrochemistry, 2023, 2 DOI:10.3389/fbael.2023.1301806 |
| 48. | Ming Feng, Xiao-li Xi, Li-wen Zhang, et al. In Situ Spectroscopic Identification and DFT-Assisted Prediction of the Valence and Structure of Tungsten Ions in a Cationic/Anionic Molten Salt System. The Journal of Physical Chemistry C, 2023, 127(6): 3142. DOI:10.1021/acs.jpcc.2c08772 |
| 49. | Hui Dang, Zhidong Chang, Hualei Zhou, et al. Extraction of lithium from the simulated pyrometallurgical slag of spent lithium-ion batteries by binary eutectic molten carbonates. International Journal of Minerals, Metallurgy and Materials, 2022, 29(9): 1715. DOI:10.1007/s12613-021-2366-3 |
| 50. | Xiaoyun Jing, Yongsong Ma, Fan Wang, et al. CO2‐Derived Oxygen‐Rich Carbon with Enhanced Redox Reactions as a Cathode Material for Aqueous Zn‐Ion Batteries. ChemistrySelect, 2022, 7(20) DOI:10.1002/slct.202201133 |
| 51. | Shuangxi Jing, Juanxiu Xiao, Yijun Shen, et al. Silicate‐Mediated Electrolytic Silicon Nanotube from Silica in Molten Salts. Small, 2022, 18(35) DOI:10.1002/smll.202203251 |
| 52. | Mingyong Wang, Handong Jiao, Zhenghao Pu, et al. Ultra‐High Temperature Molten Oxide Electrochemistry. Angewandte Chemie International Edition, 2022, 61(32) DOI:10.1002/anie.202206482 |
| 53. | Ming Feng, Li-wen Zhang, Xiao-li Xi, et al. Determination of coordination form of tungsten ion in molten alkali chloride by electrochemical method. Journal of Molecular Liquids, 2022, 346: 117062. DOI:10.1016/j.molliq.2021.117062 |
| 54. | Ming Feng, Xiao-li Xi, Li-wen Zhang, et al. Valence and coordination form transition of tungsten ions in molten alkali chlorides. Physical Chemistry Chemical Physics, 2022, 24(34): 20130. DOI:10.1039/D2CP01359B |
| 55. | Sanghyeok Im, Nathan D. Smith, Stephanie Castro Baldivieso, et al. Electrochemical recovery of Nd using liquid metals (Bi and Sn) in LiCl-KCl-NdCl3. Electrochimica Acta, 2022, 425: 140655. DOI:10.1016/j.electacta.2022.140655 |
| 56. | Seshadri Seetharaman, Lijun Wang, Haijuan Wang. Slags containing transition metal (chromium and vanadium) oxides—Conversion from ticking bombs to valuable resources: Collaborative studies between KTH and USTB. International Journal of Minerals, Metallurgy and Materials, 2022, 29(4): 750. DOI:10.1007/s12613-022-2424-5 |
| 57. | Mingyong Wang, Handong Jiao, Zhenghao Pu, et al. Ultra‐High Temperature Molten Oxide Electrochemistry. Angewandte Chemie, 2022, 134(32) DOI:10.1002/ange.202206482 |
| 58. | Gilda Aleaba, Shaghayegh Khedmatgozar Asadi, Nader Daneshvar, et al. [2, 2'-Bipyridine]-1, 1'-diium perchlorate as a new and efficient dicationic organic salt for the promotion of the synthesis of bis(4-hydroxycoumarin), 5-arylidene barbituric acid and pyrano[2, 3-d]pyrimidinone derivatives in water. Research on Chemical Intermediates, 2022, 48(5): 1953. DOI:10.1007/s11164-022-04692-y |
| 59. | Ming Feng, Xiao-Li Xi, Li-Wen Zhang, et al. Dissolution of tungsten carbide and valence analysis of tungsten ions in chloride–fluoride molten salts. Journal of Molecular Liquids, 2022, 355: 118944. DOI:10.1016/j.molliq.2022.118944 |
| 60. | Yiru Yang, Qipeng Bao, Lei Guo, et al. Numerical simulation of flash reduction in a drop tube reactor with variable temperatures. International Journal of Minerals, Metallurgy and Materials, 2022, 29(2): 228. DOI:10.1007/s12613-020-2210-1 |
| 61. | Kang Yan, Chongwei Liu, Liping Liu, et al. Pyrolysis behaviour and combustion kinetics of waste printed circuit boards. International Journal of Minerals, Metallurgy and Materials, 2022, 29(9): 1722. DOI:10.1007/s12613-021-2299-x |
| 62. | Liwen Zhang, Xiaoli Xi, Zuoren Nie. A new method for preparation of tungsten carbide powder by in situ electrochemical reduction. Electrochemistry Communications, 2022, 134: 107179. DOI:10.1016/j.elecom.2021.107179 |
| 63. | Wenxuan Qin, Xiaoli Xi, Liwen Zhang, et al. Effect of cathodic current density on the microstructure and performance of W-ZrO2 composites coating prepared in Na2WO4-WO3-ZrO2 molten salt. Surface and Coatings Technology, 2022, 440: 128497. DOI:10.1016/j.surfcoat.2022.128497 |
| 64. | Yaxin Qu, Ming Cheng, Yan Luo, et al. Removal of rare earth fission products from LiCl–KCl molten salt by sulfide precipitation. Journal of Radioanalytical and Nuclear Chemistry, 2022, 331(9): 4011. DOI:10.1007/s10967-022-08436-5 |
| 65. | Anita Zeidler, Philip S. Salmon, Takeshi Usuki, et al. Structure of molten NaCl and the decay of the pair-correlations. The Journal of Chemical Physics, 2022, 157(9) DOI:10.1063/5.0107620 |
| 66. | Ming Feng, Xiao-li Xi, Li-wen Zhang, et al. Tungsten Ion Complexes in a Cationic/Anionic Molten Salt System: Thermodynamic/Dynamic/Simulation Investigation Reveals Configuration-Induced Changes in Reaction. The Journal of Physical Chemistry C, 2022, 126(36): 15528. DOI:10.1021/acs.jpcc.2c04992 |
| 67. | Rui Yuan, Cheng Lv, Heli Wan, et al. Electrochemical behavior of vanadium ions in molten LiCl-KCl. Journal of Electroanalytical Chemistry, 2021, 891: 115259. DOI:10.1016/j.jelechem.2021.115259 |
| 68. | Shaolong Li, Yusi Che, Jianxun Song, et al. Preparation of zirconium metal through electrolysis of zirconium oxycarbonitride anode. Separation and Purification Technology, 2021, 274: 118803. DOI:10.1016/j.seppur.2021.118803 |
| 69. | Tatiana Grebennikova, Abbie N. Jones, Clint A. Sharrad. Electrochemical decontamination of irradiated nuclear graphite from corrosion and fission products using molten salt. Energy & Environmental Science, 2021, 14(10): 5501. DOI:10.1039/D1EE00332A |
| 70. | Yuan Zhong, Min Wang, Huaiyou Wang, et al. Thermodynamic description of the quaternary Mg(NO3)2–KNO3–NaNO3–LiNO3 system and investigation on the novel Mg(NO3)2 based nitrate salts with low temperature. Solar Energy Materials and Solar Cells, 2021, 230: 111148. DOI:10.1016/j.solmat.2021.111148 |
| 71. | Jinglong Liang, Rui Zhang, Hui Li, et al. The Electrochemical Mechanism of Preparing Mn from LiMn2O4 in Waste Batteries in Molten Salt. Crystals, 2021, 11(9): 1066. DOI:10.3390/cryst11091066 |
| 72. | Xianwei Su, Xiaojia Shang, Yusi Che, et al. In-situ synthesis of zirconium oxycarbide by electroreduction of ZrO2/C in molten salt. Ceramics International, 2021, 47(15): 21459. DOI:10.1016/j.ceramint.2021.04.156 |
| 73. | Shu-qiang Jiao, Ming-yong Wang, Wei-li Song. Editorial for special issue on high-temperature molten salt chemistry and technology. International Journal of Minerals, Metallurgy and Materials, 2020, 27(12): 1569. DOI:10.1007/s12613-020-2225-7 |
| 74. | Thomas Villalón. TMS 2024 153rd Annual Meeting & Exhibition Supplemental Proceedings. The Minerals, Metals & Materials Series, DOI:10.1007/978-3-031-50349-8_125 |
| 75. | Buju Guo, Yaowu Wang, Wenxiong Dong, et al. Light Metals 2025. The Minerals, Metals & Materials Series, DOI:10.1007/978-3-031-80676-6_157 |
| 76. | LingYue Song, Hui Li, Jinglong Liang. TMS 2025 154th Annual Meeting & Exhibition Supplemental Proceedings. The Minerals, Metals & Materials Series, DOI:10.1007/978-3-031-80748-0_68 |
| 77. | Jean‐Philippe Harvey, Mohamed Khalil, Jamal Chaouki. Electronic Waste. DOI:10.1002/9783527816392.ch7 |
| 78. | Samira Sokhanvaran, Eltefat Ahmadi, Natalie Wong. Proceedings of the 62nd Conference of Metallurgists, COM 2023. DOI:10.1007/978-3-031-38141-6_91 |
| Method | Melt | Cathode reaction | Anode reaction | Overall reaction | Temperature / K | Year |
| FFC | CaCl2 | TiO2+e−→Ti +2O2− | 2O2−→O2+4e− | TiO2→Ti+O2 | 1072–1123 | 2000 |
| SOM | CaCl2 –CaF2 | TiOx+2xe−→ Ti+xO2− | O2−+2H2→2H2O+2e− | 2TiOx+2xH2→ 2xH2O+2Ti | 1473–1673 | 2001 |
| MER | NaCl–KCl | Ti3++3e−→Ti | TiO2+C→Ti3++CO2+3e− or TiO2+2C→Ti3++2CO+3e− | TiO2+C→Ti+CO2 or TiO2+2C→Ti+2CO | 1073 | 2002 |
| QIT | CaF2 CaCl2 or CaF2 | TiO2+e−→Ti +2O2− | C+2O2−→CO2+4e− | TiO2+C→Ti+CO2 | 1973–2073 | 2003 |
| USTB | NaCl–KCl | Tin++ne−→Ti (n < 4) | TiCxOy→Tin++CO2+CO+ne− | TiCxOy→Ti+CO2+CO | 1073 | 2006 |
| Material | Melting point / K | Temperature change / K | Material cost / ($·kg−1) | Thermal storage cost / ($·kW−1·h−1·t−1) |
| Hitec | 415 | 473 | 0.93 | 10.7 |
| Hitec XL | 393 | 473 | 1.19 | 15.2 |
| solar salt | 493 | 473 | 0.49 | 5.8 |
| Melt | Anode reaction | Cathode reaction | Overall reaction | Reduction pathway | Temperature / K |
| NaCl–KCl | WC → W6+ + C + 6e− | W6++6e−→W | WC→W+C | W6+→W4+→W | 1023 |
| NaCl–KCl– Na2WO4 | 3WC+Na2WO4→ 4W6++3CO+Na2O+24e− | W6++6e−→W | 3WC+Na2WO4→4W+3CO+ Na2O | W6+→W | 1023 |
| NaCl–KCl–WO3 | 3WC+WO3→4W6++3CO+24e− | W6++6e−→W | 3WC+WO3→4W+3CO | W6+→W | 1023 |
| NaF–KF | WC−2e−→W2++C | W2++2e−→W | WC→W+C | W2+→W | 1073 |



