| Cite this article as: | Qian Zhao, Zhenli He, Yuehui He, Yue Qiu, Zhonghe Wang, and Yao Jiang, Porous TiFe2 intermetallic compound fabricated via elemental powder reactive synthesis, Int. J. Miner. Metall. Mater., 31(2024), No. 4, pp.764-772. https://doi.org/10.1007/s12613-023-2748-9 |
Intermetallic compounds, combining the properties of both ceramics and traditional metals, show excellent corrosion resistance in different environments and good structural stability, mainly because of the special crystal structure and chemical bond characteristics (directed covalent bonds and metallic bonds) [1–5]. Porous intermetallic compounds have received attention and were applied in the fields of filtration and separation as well as catalysis. In the field of filtration and separation, systems such as Ti–Al [6–8], Fe–Al [9–10], Ni–Al [11–13], and Fe–Si [14–16] have been systematically studied and have shown good corrosion resistance in harsh environments. With rapid industrialization, the generation of wastewater is dramatically increasing [17]. Wastewater produced from different fields shows different pH, among them, alkaline wastewater can be produced from pulp and paper, dye manufacturing, textile, etc. [18–20]. Filtration is a viable approach for wastewater treatment, which has been carried out in strong alkali solutions [21–24]. Currently, stainless steels are widely used for water treatment, but their corrosion resistances depend on the properties of passive film covering the surface [25]. For example, the passive film formed on 316L stainless steel in aggressive environments leads to a decrease in corrosion resistance [26]. Additionally, high Ni contents in 316L stainless steel increase the price and limit the application [27]. Therefore, the study of porous intermetallic compounds with lower cost and comparable corrosion resistance is necessary.
In the field of catalysis, porous intermetallic compounds are known to possess a substantial specific surface area, which results in an increased number of active sites, and thus tend to exhibit better catalytic activity [28–29]. Fe–Si [14–16] and Ti–Ni [29] systems have been shown to have commendable oxygen evolution reaction (OER) performance in acids and exceptional hydrogen evolution reaction (HER) performance in KOH, respectively. However, there are still relatively few studies on porous intermetallic catalysts. In conclusion, it is imperative to further investigate the catalytic performance of porous intermetallic compounds.
As a first-row transition metal, Fe exhibits excellent intrinsic catalytic activity [30–31]. Additionally, Fe-based porous intermetallic compounds are known for their low cost. Ti, as a corrosion-resistant metal, is suitable for filtration in various media but has a high cost [32–34]. Accordingly, the introduction of Ti to obtain porous Ti–Fe intermetallic compounds leads to enhanced pore characteristics and improved structural durability [35–36], thereby exhibiting potential for applications in filtration and separation processes.
In this work, we prepared porous TiFe2 (Fe-rich phase) intermetallic compound by the reactive synthesis of elemental powders. The formation of pore structure and phase transformation of porous TiFe2 intermetallic compound were clarified and the effects of cold pressing pressure on pore structure and mechanical properties were investigated. Besides, the corrosion characteristics and HER performance of porous TiFe2 intermetallic compound in 1 mol/L KOH were studied, which suggests that the porous TiFe2 exhibits better corrosion resistance than porous 316L stainless steel as well as good HER performance.
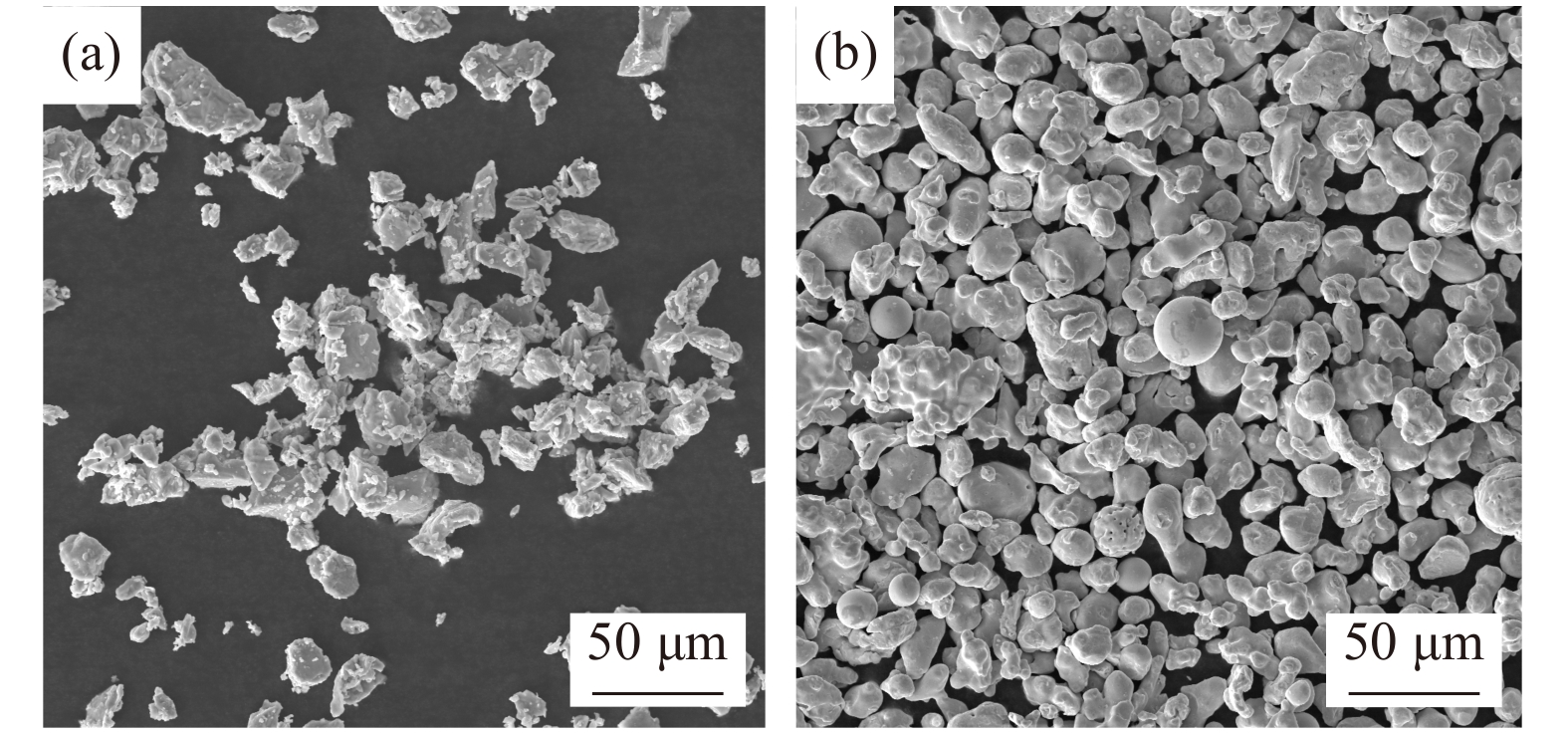
Ti powders (99.9%, median particle size (D50) = 28.7 μm) and Fe powders (99.9%, D50 = 25.9 μm) were purchased from Beijing Xing Rong Yuan Technology Co. Ltd. (Beijing, China) (Fig. 1). KOH (≥85wt%) was obtained from Sinopharm Chemical Reagent Co. Ltd. (Shanghai, China). In this work, all the chemical reagents were commercially available and were used for experiment without any further treatment.
The Ti and Fe elemental powders were thoroughly mixed in the V-type mixer for 48 h at an atomic ratio of Ti : Fe = 1:2, which is in the range of TiFe2 in the Ti–Fe phase diagram. Then, the prepared powders are placed in a vacuum furnace for loose-powder sintering. For comparison, the samples prepared by the mold press method at different pressures (50, 100, 150, and 200 MPa, denoted as TiFe2-50, TiFe2-100, TiFe2-150, and TiFe2-200, respectively) were also produced for sintering. The samples were sintered with the vacuum degree controlled at 1 × 10−2 Pa or below. The sintering process is carried out at stages of 120, 750, 900, 1100, and 1250°C, respectively, for making full use of the Kirkendall effect to promote the formation of pores, and the cooling stage was held at 900 and 600°C to reduce the thermal stress generated during the rapid cooling of the material. Porous 316L stainless steel was prepared by loose-powder sintering for comparison, the sintering process was carried out at 120 and 1100°C.
The phase composition of obtained porous TiFe2 was revealed by X-ray diffractometer (XRD, Rigaku D/Max 2500) with Cu Kα (λ = 0.15406 nm) as the radiation source. Besides, the pore structure and surface morphology of porous TiFe2 were observed using an environmental scanning electron microscope (FESEM, MIRA 3) with an energy dispersive X-ray (EDX) unit. The open porosity was determined by the Archimedes method, maximum pore size was measured by the bubble point method, and the gas permeability was determined by the gas transmission method. The mechanical properties of porous TiFe2 were determined by the three-point flexural test on electric servo-hydraulic material test system (Instron 3369, Instron Ltd., High Wycombe, UK). The sintered porous TiFe2 samples were made into rectangular shapes and used for the bending strength test, in which the span was 30 mm and the displacement rate was 0.02 mm/min.
The electrochemical measurements were carried out in a standard three-electrode cell system (CHI660D), and the electrolyte was 1 mol/L KOH solution. The saturated calomel electrode (SCE) was used as the reference and the platinum plate was used as counter electrodes. The loose-powder sintered TiFe2 was prepared as the working electrode with an apparent area of 1 cm2. Before the Tafel and electrochemical impedance spectroscopy (EIS) tests, the electrode was tested by open circuit for 1 h. The Tafel test was performed at a scan rate of 1 mV·s−1. EIS analysis was performed in a frequency range from 0.01 to 105 Hz. Linear sweep voltammetry (LSV) was carried out at a scan rate of 1 mV·s−1, and the working electrodes were activated by the LSV test several times until the curves were stable before the LSV tests. All polarization curves were corrected with the iR in this work, and all potentials in this study are converted to the reversible hydrogen electrode (RHE) reference scale by equation as follows:
| E(RHE)=E(SCE)+0.059pH+0.244 | (1) |
Fig. 2 reveals the micromorphology of loose-powder sintered TiFe2 and TiFe2-100. The loose-powder sintered TiFe2 has pores with a size of about 30 μm, while the synthesized TiFe2-100 has pores with a size of about 15 μm. Meanwhile, there are some Kirkendall pores with a size of ~2–30 μm. The synthesized loose-powder sintered TiFe2 has a higher open porosity than TiFe2-100, and they both show a smooth surface of pore structure.
Fig. 3 presents the XRD results of Ti–Fe samples sintered at 750, 900, 1100, and 1250°C, respectively. Before the sintering process, the mixed powders of Ti and Fe include the elemental Ti phase (PDF#65-9622) and the elemental Fe phase (PDF#87-0722). At 750°C, the main phases in the sintered Ti–Fe samples are Ti phase (PDF#65-9622) and Fe phase (PDF#87-0722), and the angles of Ti phase peaks in the XRD result offset in the direction of large angles, which suggests the formation of the solid solution of Fe in Ti. According to the Ti–Fe phase diagram, the solid solution phase was identified as (βTi). The equation for the diffusion reaction at 750°C stage is as follows:
| Ti + Fe →(βTi) | (2) |
At 900°C, the phases in the sintered Ti–Fe samples are composed of Ti, Fe, TiFe (PDF#65-5613), and TiFe2 (PDF#65-0602). As the diffusion reaction continues, the Ti solid solution still remains, while the Fe solid solution, TiFe, and TiFe2 phases appear after the sintering stage at 900°C. The equations for the diffusion reaction at 900°C stage are as follows:
| Ti + Fe →(βTi)+ (αFe) | (3) |
| (βTi)+ (αFe) → TiFe + TiFe2 | (4) |
At 1100°C, the elemental Ti and Fe were almost exhausted during the reaction procedure, and the phases in the sintered samples are mainly composed of TiFe and TiFe2. The inhomogeneous distribution of Ti and Fe particles in the samples causes the inhomogeneity of diffusion at low temperatures, and thus causes the formation of TiFe phase. The equation for the diffusion reaction at 1100°C stage is as follows:
| (βTi)+ (αFe) → TiFe + TiFe2 | (5) |
At 1250°C, the phases in the sintered Ti–Fe samples are basically a monolithic TiFe2 phase along with traces of TiFe phase, indicating the successful preparation of porous TiFe2 intermetallic compound. The equation for the diffusion reaction at 1250°C stage is as follows:
| (αFe) + TiFe → TiFe2 | (6) |
Fig. 4 shows the micromorphology of the loose-powder sintered TiFe2 and TiFe2-100 at different temperatures (750, 900, 1100, and 1250°C) in backscattered electron images (BSE), in which EDX analysis was carried out to determine the phases. At the sintering stage of 750°C, diffusion reaction occurs between Ti and Fe particles, and the solid solution Ti is observed between Ti particles and Fe particles. The major phases are still elemental Ti and Fe phases at this stage. When the sintering stage at 900°C finished, the diffusion reaction proceeds further, and a series of components with different Ti and Fe ratios are generated, which indicates the elemental diffusion. In this stage, a minority of TiFe2 is formed. At 1100°C, as the diffusion reaction proceeds further, the percentage of TiFe2 phase increases significantly compared to that at 900°C, and a minority of Ti and Fe solid solution phases were observed at this stage. At 1250°C, the diffusion reaction is basically complete, Ti and Fe are uniformly distributed in the porous skeleton, and there is a single TiFe2 phase with a small amount of TiFe phase in the samples. Notably, there is no difference between the steps of diffusion reactions of the loose-powder sintered TiFe2 and TiFe2-100 in different sintering stages, indicating the change in pressure had no effect on the steps of diffusion reaction.
The pore formation mechanisms of loose-powder sintered TiFe2 and TiFe2-100 exhibit certain distinctions. For the loose-powder sintered TiFe2 sample, when the temperature increases to 750°C, there is no good metallurgical bond generated. At 900°C, a small number of pores are generated inside the Fe particles, which is caused by the different diffusion rates between the Ti and Fe elements, that is, the Kirkendall effect. As the temperature increases, the Kirkendall pores participate in the total pore formation with further pore evolution in the following insulation phase. Due to the relatively minor contribution of Kirkendall pores to the overall porosity, their presence did not result in a significant increase in porosity during the subsequent insulation stage. In other words, the pores of loose-powder sintered TiFe2 are generated by a combination of the bridging effect of particles and the Kirkendall effect, in which the Kirkendall effect plays a smaller role. For the TiFe2-100, the cold forming of the powder mixture results in tighter connections between powder particles and a lower initial open porosity. At 900 and 1100°C, the larger contact area between powder particles causes a significant increase in element diffusion and the generation of a large number of Kirkendall pores inside the Fe particles. During the final stage of insulation, the Kirkendall pores participate in the formation of the pore structure as a result of surface diffusion. Since the number of Kirkendall pores is much higher than that of the loose-powder sintered TiFe2 samples, the open porosity depends not only on the bridging effect but also on the Kirkendall effect. In the case of TiFe2-100, the contribution of Kirkendall pores to the total porosity is significantly higher than that of loose-powder sintered TiFe2, which is responsible for the increase in porosity observed after heat treatment at both 1100 and 1250°C.
The changing trends of open porosity of loose-powder sintered TiFe2 and TiFe2-100 suggest the same speculation as above (Fig. 5(a)). In the reactive synthesis process, the open porosity of the loose-powder sintered TiFe2 samples basically decreases linearly with the increase of temperature and reaches the open porosity of 56.4% after the 1250°C stage, which is caused by the surface diffusion behavior and the decrease of specific surface area. For the TiFe2-100, the open porosity decreases linearly when the stage holds a temperature below 900°C and increases linearly when the temperature gets higher than 900°C, reaching the final value of 38.0%. The diffusion reaction is slow and the Kirkendall effect is hardly involved in the formation of pores before the 900°C stages, as a result, the open porosity decreases rapidly; when the temperature is higher than 900°C, a large number of Kirkendall pores are generated and make a significant contribution to the total porosity. The results suggest that the variation of pressing pressure has an effect on the formation of the pore structure of TiFe2, therefore, the pore structure of porous TiFe2 can be well controlled by controlling the pressing pressure and sintering temperature.
The mixed TiFe2 powders were pressed into compacts under different pressures and the open porosity, maximum pore size, and gas permeability of them were measured. As shown in Fig. 5(b) and (c), the open porosity, maximum pore size, and gas permeability of TiFe2 samples decrease linearly with the increase of pressure of cold pressing, which is due to the reduction of interstitial space between particles. Meanwhile, the open porosity, maximum pore size, and gas permeability of the loose-powder sintered TiFe2 were much larger than those of the TiFe2 compacts with pressure. Studies have already indicated that the open porosity and the average pore size of the porous samples affect the bending strength of the samples [1]. Therefore, the bending strength of porous TiFe2 samples increases as the pressure increases, which can be attributed to the simultaneous decrease in open porosity and the average pore size (Fig. 5(d)).
Fig. 6(a) shows the potentiodynamic polarization curve measured in 1 mol/L KOH, and the same curve of porous 316L stainless steel was measured for comparison. The wide range of passive regions observed in the potentiodynamic polarization curves suggests the strong passivation ability of porous TiFe2 intermetallic and porous 316L stainless steel. The passivation region of porous TiFe2 is significantly wider than that of porous 316L stainless steel, indicating excellent corrosion resistance of porous TiFe2 in KOH solution. The corrosion current density (Icorr) values were determined by the Tafel curves. The porous TiFe2 samples had a more positive corrosion potential (Ecorr) than porous 316L stainless steel, while porous 316L stainless steel had a higher Icorr, indicating that the porous TiFe2 is thermodynamically more difficult to corrode, while kinetically corrosion is more retarded in KOH (Table 1).
Fig. 6(b) and (c) shows the Nyquist diagrams and the equivalent circuits of porous TiFe2 and 316L stainless steel. In the equivalent circuits, Rs represents the solution resistance, CPE1 and CPE2 represent the constant phase element, and Rf represents the passive film resistance. The impedance spectra show different characteristics: for porous TiFe2, the Nyquist plot shows a semicircle, which reflects the charge transfer process; the Nyquist plot of porous 316L stainless steel exhibits a high-frequency semicircle and a low-frequency oblique line, and the oblique line is related to the concentration polarization dominated by both the ion diffusion rate and the activation energy of the electrochemical reaction [37]. In general, the charge transfer resistance (Rct) is usually utilized to assess the corrosion rate, and a higher value of Rct represents a lower corrosion rate [38]. According to the electrochemical parameters (Table 2), TiFe2 shows a higher Rct value than that of 316L stainless steel, indicating lower corrosion rate of TiFe2. Fig. 6(d) shows the open-circuit potential curve of loose-powder sintered porous TiFe2 for 24 h. At the onset of the curve, noticeable oscillations are observed owing to the unsteady nature of the surface; however, prompt stabilization is subsequently achieved. There is no significant change observed, indicating the stability of porous TiFe2 in KOH.
| Sample | Ecorr / V | Icorr / (mA·cm−2) |
| Porous TiFe2 | −0.858 | 0.269 |
| Porous 316L stainless steel | −1.108 | 0.314 |
| Sample | Rs / (Ω·cm2) | CPE1 / (F·cm−2) | Rct / (Ω·cm2) | CPE2 / (F·cm−2) | Rf / (Ω·cm2) |
| Porous TiFe2 | 4.80 | 0.0039 | 452.30 | — | — |
| Porous 316L stainless steel | 2.38 | 0.0050 | 131.80 | 0.048 | 1862.87 |
The evolution of pore structure and surface morphology before and after the Tafel tests are also investigated (Fig. 7(a)–(d)). The surface of the TiFe2 samples is corroded and a layer of iron oxyhydroxide nanosheets grows on the surface of the porous TiFe2 samples. Studies of TiNi suggest a similar phenomenon [39]. In the process of polarization, Ti and Fe elements transition to the oxidation state due to the loss of electrons, leading to the formation of a Ti-rich oxide layer and different types of titanates by Ti and the formation of FeOOH due to the oxidation of Fe, and then the resultant deposits on the sample surface and prevents the further corrosion of KOH [39], which is related to the good corrosion resistance of TiFe2 [39]. Meanwhile, there is no change in the pore structure of porous TiFe2 samples, indicating good structural stability. X-ray photoelectron spectroscopy (XPS) was employed to investigate the chemical composition and valence state of porous TiFe2 samples before and after the Tafel test. In the high-resolution Ti 2p spectra (Fig. 7(e)), the peaks at 454.68 and 460.83 eV correspond to Ti0, and the peaks at 458.68 and 464.83 eV, correspond to Ti4+ retained after the Tafel test. In the high-resolution Fe 2p spectra (Fig. 7(f)), the peaks at 711.93 and 724.43 eV represent the characteristic Fe 2p3/2 and Fe 2p1/2 of Fe3+ before and after Tafel test, indicating the dominant oxidation states of Fe, which is consistent with the previous inference.
To assess the HER activity of nanosheets on porous TiFe2 intermetallic electrodes, LSV was carried out in 1 mol/L KOH solution with a standard three-electrode configuration. Fig. 8(a) presents the LSV curve, in which porous TiFe2 exhibited overpotentials of 220.6 and 295.6 mV at 10 and 100 mA·cm−2, respectively, and a Tafel slope of 105.6 mV·dec−1 (Fig. 8(b)), which is not comparable to Pt-based catalysts but still presents some prospects regarding its implementation as a catalyst.
In summary, porous TiFe2 intermetallic compounds are prepared by the reactive synthesis of elemental powders. The bridging effect and the Kirkendall effect work in combination to participate in the formation of the pore structure of the porous TiFe2 intermetallics. The corrosion behavior of porous TiFe2 in alkali solution suggested higher Ecorr, lower Icorr, and wider passivation regions, and thus exhibited better corrosion resistance than porous 316L stainless steel. A layer of nanosheets is formed on the surface after the potentiodynamic polarization test, which hinders further corrosion. Besides, porous TiFe2 electrode exhibited overpotentials of 220.6 and 295.6 mV at 10 and 100 mA·cm−2, respectively. Our study on porous TiFe2 intermetallics has demonstrated its good corrosion resistance and HER performance, which demonstrates the potential of Fe-based intermetallics for filter separation and catalytic applications in alkaline environments.
This work was financially supported by the National Natural Science Foundation of China (No. 51971251).
All the authors declare that there is no conflict of interest regarding the publication of this paper.
| [1] |
Y. Jiang, Y.H. He, and H.Y. Gao, Recent progress in porous intermetallics: Synthesis mechanism, pore structure, and material properties, J. Mater. Sci. Technol., 74(2021), p. 89. DOI: 10.1016/j.jmst.2020.10.007
|
| [2] |
J.J. Wan, Z.M. Zhang, Y.M. Wang, et al., Synergistic covalent-and-supramolecular polymers connected by [2]pseudorotaxane moieties, Chem. Commun., 57(2021), No. 60, p. 7374. DOI: 10.1039/D1CC02873A
|
| [3] |
Y.L. Zhang, A.H. Feng, S.J. Qu, J. Shen, and D.L. Chen, Microstructure and low cycle fatigue of a Ti2AlNb-based lightweight alloy, J. Mater. Sci. Technol., 44(2020), p. 140. DOI: 10.1016/j.jmst.2020.01.032
|
| [4] |
Z.C. Shang, X.P. Cai, H. Wang, et al., High temperature anti-oxidation and filtration behavior of micro/nano-scale porous CoAl intermetallic synthesized via rapid thermal explosion, Corros. Sci., 219(2023), art. No. 111216. DOI: 10.1016/j.corsci.2023.111216
|
| [5] |
Z.C. Shang, X.P. Cai, X.Y. Jiao, et al., 3D microstructure and anti-oxidation behavior of porous CoAl intermetallic fabricated by rapid thermal explosion, Corros. Sci., 208(2022), art. No. 110715. DOI: 10.1016/j.corsci.2022.110715
|
| [6] |
X.Y. Jiao, P.Z. Feng, J.Z. Wang, X.R. Ren, and F. Akhtar, Exothermic behavior and thermodynamic analysis for the formation of porous TiAl3 intermetallics sintering with different heating rates, J. Alloys Compd., 811(2019), art. No. 152056. DOI: 10.1016/j.jallcom.2019.152056
|
| [7] |
Y.H. He, Y. Jiang, N.P. Xu, et al., Fabrication of Ti–Al micro/nanometer-sized porous alloys through the Kirkendall effect, Adv. Mater., 19(2007), No. 16, p. 2102. DOI: 10.1002/adma.200602398
|
| [8] |
G.L. Hao, H. Wang, and X.Y. Li, Novel double pore structures of TiAl produced by powder metallurgy processing, Mater. Lett., 142(2015), p. 11. DOI: 10.1016/j.matlet.2014.11.135
|
| [9] |
H. Sina, J. Corneliusson, K. Turba, and S. Iyengar, A study on the formation of iron aluminide (FeAl) from elemental powders, J. Alloys Compd., 636(2015), p. 261. DOI: 10.1016/j.jallcom.2015.02.132
|
| [10] |
G. Chen, K.D. Liss, C. Chen, Y.H. He, X.H. Qu, and P. Cao, Porous FeAl alloys via powder sintering: Phase transformation, microstructure and aqueous corrosion behavior, J. Mater. Sci. Technol., 86(2021), p. 64. DOI: 10.1016/j.jmst.2021.01.029
|
| [11] |
Y.M. Shu, A. Suzuki, N. Takata, and M. Kobashi, Fabrication of porous NiAl intermetallic compounds with a hierarchical open-cell structure by combustion synthesis reaction and space holder method, J. Mater. Process. Technol., 264(2019), p. 182. DOI: 10.1016/j.jmatprotec.2018.09.010
|
| [12] |
T. Ide, M. Tane, and H. Nakajima, Fabrication of lotus-type porous NiAl and Ni3Al intermetallic compounds, Solid State Phenom., 124-126(2007), p. 1721. DOI: 10.4028/www.scientific.net/SSP.124-126.1721
|
| [13] |
L. Wu, Y.H. He, T. Lei, et al., The stability of hydrogen evolution activity and corrosion behavior of porous Ni3Al–Mo electrode in alkaline solution during long-term electrolysis, Energy, 67(2014), p. 19. DOI: 10.1016/j.energy.2014.02.033
|
| [14] |
B.T. Shen, Y.H. He, Z.H. Wang, L.P. Yu, Y. Jiang, and H.Y. Gao, Reactive synthesis of porous FeSi intermetallic compound, J. Alloys Compd., 826(2020), art. No. 154227. DOI: 10.1016/j.jallcom.2020.154227
|
| [15] |
B.T. Shen, Y.H. He, W.H. Li, et al., Insight into electrochemical performance of porous Fe x Si y intermetallic anode for zinc electrowinning, Mater. Des., 191(2020), art. No. 108645. DOI: 10.1016/j.matdes.2020.108645
|
| [16] |
B.T. Shen, Y.H. He, Z.L. He, Z.H. Wang, Y. Jiang, and H.Y. Gao, Porous Fe5Si3 intermetallic anode for the oxygen evolution reaction in acidic electrolytes, J. Colloid Interface Sci., 605(2022), p. 637. DOI: 10.1016/j.jcis.2021.07.127
|
| [17] |
S. Malik, S. Kishore, S. Prasad, and M.P. Shah, A comprehensive review on emerging trends in industrial wastewater research, J. Basic Microbiol., 62(2022), No. 3-4, p. 296. DOI: 10.1002/jobm.202100554
|
| [18] |
V. Kumar and S.K. Dwivedi, A review on accessible techniques for removal of hexavalent Chromium and divalent Nickel from industrial wastewater: Recent research and future outlook, J. Cleaner Prod., 295(2021), art. No. 126229. DOI: 10.1016/j.jclepro.2021.126229
|
| [19] |
A.V. Baskar, N. Bolan, S.A. Hoang, et al., Recovery, regeneration and sustainable management of spent adsorbents from wastewater treatment streams: A review, Sci. Total Environ., 822(2022), art. No. 153555. DOI: 10.1016/j.scitotenv.2022.153555
|
| [20] |
R.M. Jain, K.H. Mody, J. Keshri, and B. Jha, Biological neutralization and biosorption of dyes of alkaline textile industry wastewater, Mar. Pollut. Bull., 84(2014), No. 1-2, p. 83. DOI: 10.1016/j.marpolbul.2014.05.033
|
| [21] |
J.H. Jeon, A.B.C. Sola, J.Y. Lee, and R.K. Jyothi, Hydrometallurgical process development to recycle valuable metals from spent SCR deNO X catalyst, Sci. Rep., 11(2021), No. 1, art. No. 22131. DOI: 10.1038/s41598-021-01726-0
|
| [22] |
A.V. Boyarintsev, S.I. Stepanov, G.V. Kostikova, V.I. Zhilov, A.M. Safiulina, and A.Y. Tsivadze, Separation and purification of elements from alkaline and carbonate nuclear waste solutions, Nucl. Eng. Technol., 55(2023), No. 2, p. 391. DOI: 10.1016/j.net.2022.09.030
|
| [23] |
A.A. Chichirov, N.D. Chichirova, A.A. Filimonova, A.I. Minibaev, and R.V. Buskin, Laboratory investigations of processing highly mineralized alkali solutions by means of electromembrane technology, Therm. Eng., 66(2019), No. 7, p. 527. DOI: 10.1134/S0040601519070036
|
| [24] |
T. Hua, R.J. Haynes, and Y.F. Zhou, Removal of Al, Ga, As, V and Mo from alkaline wastewater using pilot-scale constructed wetlands, Environ. Sci. Pollut. Res. Int., 26(2019), No. 34, p. 35121. DOI: 10.1007/s11356-019-06490-3
|
| [25] |
G. Tranchida, M. Clesi, F. Di Franco, F. Di Quarto, and M. Santamaria, Electronic properties and corrosion resistance of passive films on austenitic and duplex stainless steels, Electrochim. Acta, 273(2018), p. 412. DOI: 10.1016/j.electacta.2018.04.058
|
| [26] |
N. Jeyaprakash, C.H. Yang, S.S. Karuppasamy, and M. Duraiselvam, Stellite 6 cladding on AISI type 316L stainless steel: Microstructure, nanohardness and corrosion resistance, Trans. Indian Inst. Met., 76(2023), No. 2, p. 491. DOI: 10.1007/s12666-022-02731-1
|
| [27] |
A. Sharma, S. Shukla, M. Thombre, A. Bansod, and S. Untawale, An investigation of the effect of sensitization on the metallurgical characteristics of dissimilarly welded austenitic–ferritic stainless steel, Anti-Corros. Meth. Mater., 70(2023), No. 6, p. 361. DOI: 10.1108/ACMM-04-2023-2797
|
| [28] |
R.R. Song, J.H. Han, M. Okugawa, et al., Ultrafine nanoporous intermetallic catalysts by high-temperature liquid metal dealloying for electrochemical hydrogen production, Nat. Commun., 13(2022), No. 1, art. No. 5157. DOI: 10.1038/s41467-022-32768-1
|
| [29] |
Y. Qiu, Z.L. He, Y.H. He, Q. Zhao, Z.H. Wang, and Y. Jiang, Porous TiNi3-based intermetallics as active and robust monolith catalysts for hydrogen evolution, Chem. Commun., 58(2022), No. 100, p. 13943. DOI: 10.1039/D2CC05574K
|
| [30] |
J. Sun, N.K. Guo, T.S. Song, et al., Revealing the interfacial electron modulation effect of CoFe alloys with CoC X encapsulated in N-doped CNTs for superior oxygen reduction, Adv. Powder Mater., 1(2022), No. 3, art. No. 100023. DOI: 10.1016/j.apmate.2021.11.009
|
| [31] |
J. Abed, S. Ahmadi, L. Laverdure, et al. , In situ formation of nano Ni–Co oxyhydroxide enables water oxidation electrocatalysts durable at high current densities, Adv. Mater., 33(2021), No. 45, art. No. 2103812. DOI: 10.1002/adma.202103812
|
| [32] |
A.I. Zhevnovatyi and G.F. Shenberg, Study of the production technology of porous titanium tubes, Sov. Powder Metall. Met. Ceram., 4(1965), No. 2, p. 95. DOI: 10.1007/BF00777009
|
| [33] |
P.S. Liu and K.M. Liang, Review Functional materials of porous metals made by P/M, electroplating and some other techniques, J. Mater. Sci., 36(2001), No. 21, p. 5059. DOI: 10.1023/A:1012483920628
|
| [34] |
Z.D. Lin, K.J. Song, and X.H. Yu, A review on wire and arc additive manufacturing of titanium alloy, J. Manuf. Process., 70(2021), p. 24. DOI: 10.1016/j.jmapro.2021.08.018
|
| [35] |
J.Z. Niu, G.Q. Dai, Y.H. Guo, et al., Microstructure and mechanical properties of B modified Ti–Fe alloy manufactured by casting, forging and laser melting deposition, Composites Part B, 216(2021), art. No. 108854. DOI: 10.1016/j.compositesb.2021.108854
|
| [36] |
J.J. Noël, N. Ebrahimi, and D.W. Shoesmith, Corrosion of titanium and titanium alloys, [in] K. Wandelt, ed., Encyclopedia of Interfacial Chemistry : Surface Science and Electrochemistry, Elsevier, Amsterdam, 2018, p. 192.
|
| [37] |
Y. Xu, Y.L. Huang, F.F. Cai, D.Z. Lu, and X.T. Wang, Study on corrosion behavior and mechanism of AISI 4135 steel in marine environments based on field exposure experiment, Sci. Total Environ., 830(2022), art. No. 154864. DOI: 10.1016/j.scitotenv.2022.154864
|
| [38] |
C.X. Yi and B.F. Zhu, Corrosion inhibition effect of 2-hydroxy phosphonoacetic acid and pyrophosfate on Q235 steel, electrochemical noise and EIS analysis, Int. J. Electrochem. Sci., 14(2019), No. 7, p. 6759. DOI: 10.20964/2019.07.28
|
| [39] |
Y. Zhao, L. Bai, Y.H. Sun, et al., Low-temperature alkali corrosion induced growth of nanosheet layers on NiTi alloy and their corrosion behavior and biological responses, Corros. Sci., 190(2021), art. No. 109654. DOI: 10.1016/j.corsci.2021.109654
|
| [1] | Shaorou Ke, Yajing Zhao, Xin Min, Yanghong Li, Ruiyu Mi, Yangai Liu, Xiaowen Wu, Minghao Fang, Zhaohui Huang. Highly mass activity electrocatalysts with ultralow Pt loading on carbon black for hydrogen evolution reaction [J]. International Journal of Minerals, Metallurgy and Materials, 2025, 32(1): 182-190. DOI: 10.1007/s12613-024-2912-x |
| [2] | Duanhao Cao, Xiaofeng Ma, Yipeng Zhang, La Ta, Yakun Yang, Chao Xu, Feng Ye, Jianguo Liu. Highly dispersed NiMo@rGO nanocomposite catalysts fabricated by a two-step hydrothermal method for hydrogen evolution [J]. International Journal of Minerals, Metallurgy and Materials, 2023, 30(12): 2432-2440. DOI: 10.1007/s12613-023-2677-7 |
| [3] | Xinzhuo Hu, Zhe Liu, Yi Feng, Yongfeng Zhang, Zhe Li, Zhennan Chen, Jing Mao, Jing Yang, Hui Liu, Pengfei Yin, Lei Cui, Xiwen Du. Mechanically mixing copper and silver into self-supporting electrocatalyst for hydrogen evolution [J]. International Journal of Minerals, Metallurgy and Materials, 2023, 30(10): 1906-1913. DOI: 10.1007/s12613-023-2695-5 |
| [4] | Masoud Sabzi, Sadegh Moeini Far, Saeid Mersagh Dezfuli. Effect of melting temperature on microstructural evolutions, behavior and corrosion morphology of Hadfield austenitic manganese steel in the casting process [J]. International Journal of Minerals, Metallurgy and Materials, 2018, 25(12): 1431-1438. DOI: 10.1007/s12613-018-1697-1 |
| [5] | Saeid Mersagh Dezfuli, Ali Shanaghi, Saeid Baghshahi. Effect of Al2O3 and Y2O3 on the corrosion behavior of ZrO2-benzotriazole nanostructured coatings applied on AA2024 via a sol-gel method [J]. International Journal of Minerals, Metallurgy and Materials, 2018, 25(11): 1344-1353. DOI: 10.1007/s12613-018-1688-2 |
| [6] | Bao-biao Yu, Hong Yan, Qing-jie Wu, Zhi Hu, Fan-hui Chen. Microstructure and corrosion behavior of Al3Ti/ADC12 composite modified with Sr [J]. International Journal of Minerals, Metallurgy and Materials, 2018, 25(7): 840-848. DOI: 10.1007/s12613-018-1633-4 |
| [7] | Mostafa Amirjan, Mansour Bozorg. Properties and corrosion behavior of Al based nanocomposite foams produced by the sintering-dissolution process [J]. International Journal of Minerals, Metallurgy and Materials, 2018, 25(1): 94-101. DOI: 10.1007/s12613-018-1551-5 |
| [8] | P. Laxman Mani Kanta, V. C. Srivastava, K. Venkateswarlu, Sharma Paswan, B. Mahato, Goutam Das, K. Sivaprasad, K. Gopala Krishna. Corrosion behavior of ultrafine-grained AA2024 aluminum alloy produced by cryorolling [J]. International Journal of Minerals, Metallurgy and Materials, 2017, 24(11): 1293-1305. DOI: 10.1007/s12613-017-1522-2 |
| [9] | Se-fei Yang, Ying Wen, Pan Yi, Kui Xiao, Chao-fang Dong. Effects of chitosan inhibitor on the electrochemical corrosion behavior of 2205 duplex stainless steel [J]. International Journal of Minerals, Metallurgy and Materials, 2017, 24(11): 1260-1266. DOI: 10.1007/s12613-017-1518-y |
| [10] | He-rong Zhou, Xiao-gang Li, Chao-fang Dong, Kui Xiao, Tai Li. Corrosion behavior of aluminum alloys in Na2SO4 solution using the scanning electrochemical microscopy technique [J]. International Journal of Minerals, Metallurgy and Materials, 2009, 16(1): 84-88. DOI: 10.1016/S1674-4799(09)60014-5 |
| 1. | Zixuan Pang, Zhichao Shang, Xuewei Xu, et al. Rapid fabrication and corrosion behavior of 3D-porous Cu-Al-Si compounds via low-energy self-exothermic reaction. Journal of Alloys and Compounds, 2025, 1010: 177198. DOI:10.1016/j.jallcom.2024.177198 |
| 2. | Yonghao Yu, Dapeng Zhou, Lei Qiao, et al. Highly Porous Co-Al Intermetallic Created by Thermal Explosion Using NaCl as a Space Retainer. Materials, 2024, 17(17): 4380. DOI:10.3390/ma17174380 |
| Sample | Ecorr / V | Icorr / (mA·cm−2) |
| Porous TiFe2 | −0.858 | 0.269 |
| Porous 316L stainless steel | −1.108 | 0.314 |
| Sample | Rs / (Ω·cm2) | CPE1 / (F·cm−2) | Rct / (Ω·cm2) | CPE2 / (F·cm−2) | Rf / (Ω·cm2) |
| Porous TiFe2 | 4.80 | 0.0039 | 452.30 | — | — |
| Porous 316L stainless steel | 2.38 | 0.0050 | 131.80 | 0.048 | 1862.87 |

